Location: Home >> Detail
TOTAL VIEWS
Pharm Front. 2019;1:e190004. https://doi.org/10.20900/pf20190004
1 Pharmaceutical Education Center, Yokohama University of Pharmacy, 601 Matano-cho, Totsuka-ku, Yokohama-shi, Kanagawa 245-0066, Japan
2 Research Organization of Bioactivity, Shinanomachi House #202, 18 Shinanomachi, Shinjuku-ku, Tokyo 160-0016, Japan
3 Omotesando Helene Clinic, 3F Aoyama OHMOTO Building, 5-9-15 Minamiaoyama, Minato-ku, Tokyo 170-0062, Japan
4 GHM Co., Ltd., 3-11-2 Higashi-cho, Koganei-shi, Tokyo 184-0011, Japan
* Correspondence: Katsuaki Dan, Tel.: +81-3-5379-2522; Fax: +81-3-5372-2522.
This article belongs to the Virtual Special Issue "The Evolving Role of Natural Products in Drug Discovery"
Background: Toleaf is a cold abstraction extract from the Gynura procumbens (Lour.) Merr. plant. Compounds extracted from G. procumbens exert anti-cancer effects against various cancers. However, detailed mechanisms underlying these actions remain unclear. Previous research indicated that G. procumbens ethanol extracts also have immunomodulatory properties. Therefore, we examined the relationship between Toleaf’s anti-cancer effects and immune system responses in vitro and ex vivo.
Methods: Effects of Toleaf on immune response were assessed using ELISA, RT-PCR, and flow cytometry to measure IFN-γ, IL-2, IL-4, and IL-12 expression in primary splenic cells obtained from 6-week-old male BALB/c or C57BL/6 mice and in T lymphocytes isolated from mouse splenic cells. Toleaf’s anti-cancer and T-cell activation effects were evaluated by determining CT-26, EL-4, and B16 cancer cell viability.
Results: Toleaf treatment increased splenic cell IFN-γ production and CD4+ T lymphocyte IL-2, IL-4, and IL-12-mRNA levels. All three cancer cell lines exhibited decreased viability after exposure to Toleaf-treated T lymphocytes. However, Toleaf did not exert direct cytotoxic effects on these cells.
Conclusions: Toleaf activates type 1 and 2 T helper responses, suggesting that its anti-cancer effects are associated with CD4+ T lymphocyte activation.
CD4+ T helper cell (Th) response stimulation is important in cancer immunity. Cancer immunotherapy has focused on eliciting cytotoxic T-cell (CTL) responses, with CD4+ Th cells only recently being recognized as central to immune response development. CD4+ Th cells operate by activating antigen-specific effector cells and recruiting innate immune system cells [1].
The ability of natural plant products to elicit anti-tumor effects through immune system modulation is widely investigated. However, majority of the studies have been limited to an agent’s effect in one tumor model, with few studies investigating effects across multiple cancer models [2–5].
Toleaf (Gynura procumbens (Lour.) Merr.) is widely used for treating numerous ailments [6,7]. G. procumbens leaf extract inhibits breast cancer cell line growth, whereas an ethanolic leaf extract exhibits anti-proliferative activity in a 7,12-dimethylbenz(a)anthracene-induced liver-cancer rat model [7,8]. G. procumbens ethanol extract has immunomodulatory effects [9].
Conversely, a Houttuynia cordata extract has been employed as a similar functional food in Japan. H. cordata extract inhibits human breast cancer [10], primary colorectal cancer [11], and colon adenocarcinoma cell proliferation [12]. Polysaccharides in H. Cordata extract have been shown to be effective in Lung cancer cells [13] and to induce apoptosis in melanoma [14].
We determined the relationship between the immune system and Toleaf-mediated anti-tumor effects using H. cordata as a reference for a similar functional food. We evaluated IFN secretion and several cytokine mRNA expression levels in naïve mouse splenic cells and CD4+ T lymphocytes. We examined direct cytotoxic effects of Toleaf and Toleaf-treated CD4+ T lymphocytes on several cancer cell lines. CD4+ T lymphocytes co-cultured with Toleaf significantly enhanced cytotoxicity.
Six-week-old male BALB/c and C57BL/6 mice were purchased from Charles River Laboratories Japan, Inc. (Kanagawa, Japan). MHC haplotypes were H-2d and H-2b for BalB/c and C57BL/6 mice, respectively. Primary splenic cells obtained from these mice under anesthesia [15] were cultured in Roswell Park Memorial Institute (RPMI) medium (Wako, Osaka, Japan) containing 10% fetal bovine serum (FBS; Carson, CA, USA), penicillin (100 U/mL), streptomycin (100 μg/mL), kanamycin (10 μg/mL), and L-glutamine at 37 °C in 5% CO2. All experiments were approved by the Institutional Animal Care and Use Committee of Omotesando Helene Clinic (Permit Number: ROBA#201704002, 31-03-2017) and performed in accordance with Research Organization of Bioactivity animal ethics guidelines.
Cancer Cell LinesCT-26 cells, from a mouse colon adenocarcinoma (H-2d); EL-4 cells, from a mouse T lymphocyte lymphoma (H-2b); and B16-F10 cells, from a mouse skin melanoma (H-2b), were purchased from ATCC® (Tokyo, Japan). CT-26 cells were grown in Dulbecco’s modified Eagle’s medium (Wako, Osaka, Japan), whereas EL-4 and B16 cells were grown in RPMI. Both media were supplemented with 10% FBS, penicillin (100 U/mL), streptomycin (100 μg/mL), kanamycin (10 μg/mL), and L-glutamine. All cells were maintained at 37 °C in 5% CO2. These cancer cell lines were chosen as a typical cell of solid body cancer, blood systems cancer and epidermal carcinoma.
Medicinal PlantsToleaf and H. cordata were obtained from GHM Co., Ltd. (Tokyo, Japan). Toleaf leaves were dried using a low-temperature dehumidification drying method and broken into small pieces, after which water was added. The components of the Toleaf extract have not been identified, but have been shown to contain flavonoids [16]. These leaves were dissolved in 30% ethanol at 333 mg/mL (undiluted concentration) and diluted with culture medium to achieve a final concentration of 0.0333 mg/mL. H. cordata leaves were extracted using 30% ethanol and diluted using culture medium to achieve similar concentration. Various medicinal plant extract cytotoxicities at different concentrations were evaluated using cell viability assays; the least toxic concentration (0.0333 mg/mL) was selected for further investigations (Figure 1). It was also the similar viability about EL-4, B-16-F10 and human fibroblast cells (data not shown). Even if high concentration Toleaf processing beyond 0.333 mg/mL was examined, the viability didn't fall to less than 0.69 (Abs: 450 nm) in any considered cells.
Explant mouse splenic cells treated with medicinal plant extracts were incubated for 16 h. Detected supernatant IFN-γ levels were quantified using a Mouse IFN-γ Quantikine ELISA Kit (R&D Systems, Inc., Minneapolis, MN, USA).
Reverse-Transcription Polymerase Chain Reaction (RT-PCR)TRIzol reagent (Life Technologies, CA, USA) was used to extract total RNA, which was reverse-transcribed into cDNA using PrimeScript RT Master Mix (TaKaRa, Shiga, Japan) and amplified using SYBR Premix EX Taq II (TaKaRa). Reactions were performed in a final volume of 50 μL (25 μL 2× SYBR Mix, 1 μL cDNA, 2 μL primer pair mixture (5 pmol/μL of each primer), and 22 μL H2O). PCR was performed under the following conditions: 95 °C for 30 s, followed by 50 cycles of 95 °C for 5 s and 60 °C for 30 s. Relative gene expression was calculated using the ΔΔCt method; the housekeeping gene GAPDH was used for normalization. Primers used in RT-PCR are shown in Table 1. Differences were considered to be significant when Ct was >2.
Macrophage Microsphere Phagocytosis DetectionMacrophages were isolated from mouse intraperitoneal cavities [17]. Isolated macrophages were treated with Toleaf/H. cordata extract for 1 h, followed by incubation with Fluoresbrite® Carboxylate 0.75 Micron Microspheres (Polysciences Inc., PA, USA) for 8 h. Microsphere fluorescence was detected using fluorescence microscopy (BZ-X710; KEYENCE, Osaka, Japan).
CD4+ T-Cell Isolation and Flow CytometryCD4+ T-cells were isolated from mouse splenic cell suspensions via magnetically activated cell sorting (MACS) using a CD4+ T-cell Isolation Kit (Miltenyi Biotec, Surrey, UK). Isolated T-cells treated with Toleaf/H. cordata extracts for 7 days were treated with monoclonal phycoerythrin (PE)-conjugated rat anti-mouse IFN-γ (catalog no. 562020; BD Biosciences, CA, USA) and monoclonal PE rat anti-mouse IL-4 (catalog no. 562044; BD Biosciences, CA, USA) antibodies to detect IFN-γ- and IL-4–producing T-cells, respectively. Cells were analyzed using a Muse® Cell Analyzer (Millipore, MA, USA).
Plant Extract Anti-Cancer Effect Assessment via Cell Viability AssayCell viability was determined by detecting the presence of cytosolic lactate dehydrogenase (LDH) presence in the supernatant using a LDH Cytotoxicity Detection Kit (TaKaRa). Results are presented as a percentage of the corresponding control. To measure direct cytotoxic effects of Toleaf and H. cordata extracts on cancer cells, CT-26, EL-4, and B16 cells were seeded in 96-well plates at 2 × 105 cells/well and cultured for 24 h. Cells were then treated with 1000-fold diluted Toleaf/H. cordata extract in cell-adapted culture medium for 7 days, at which point supernatants were collected for cell viability measurements. To evaluate the indirect effects of Toleaf and H. cordata extracts on cancer cells via T lymphocyte activation, isolated T lymphocytes were treated with 1000-fold diluted Toleaf/H. cordata in cell-adapted culture medium for 7 days. These T lymphocytes, at 2 × 106 cells/well, were then co-cultured with 2 × 105 CT-26, EL-4/B16 cells/well in 96-well plates for 7 days.
Statistical AnalysisData were expressed as means ± standard deviation from three individual experiments. Between-group differences were assessed using one-way analysis of variance followed by Tukey’s post hoc test using IBM SPSS Statistics Base software. P values of 0.01 or 0.001 were considered statistically significant.
Immunostimulatory effects of Toleaf and H. cordata extracts on mouse naïve splenic cells were assessed by evaluating IFN-γ secretion. IFN-γ is an important effector molecule of anti-tumor immunity [18] and plays a crucial role in suppressing tumor growth [19]. Therefore, we measured supernatant IFN-γ levels using ELISA for determining whether Toleaf extract induces immune responses in mouse splenic cells; it promoted significant mouse splenic cell IFN-γ secretion, whereas H. cordata extract inhibited it (Figure 2). This phenomenon was commonly observed in cells from BALB/c and C57BL/6 mice.
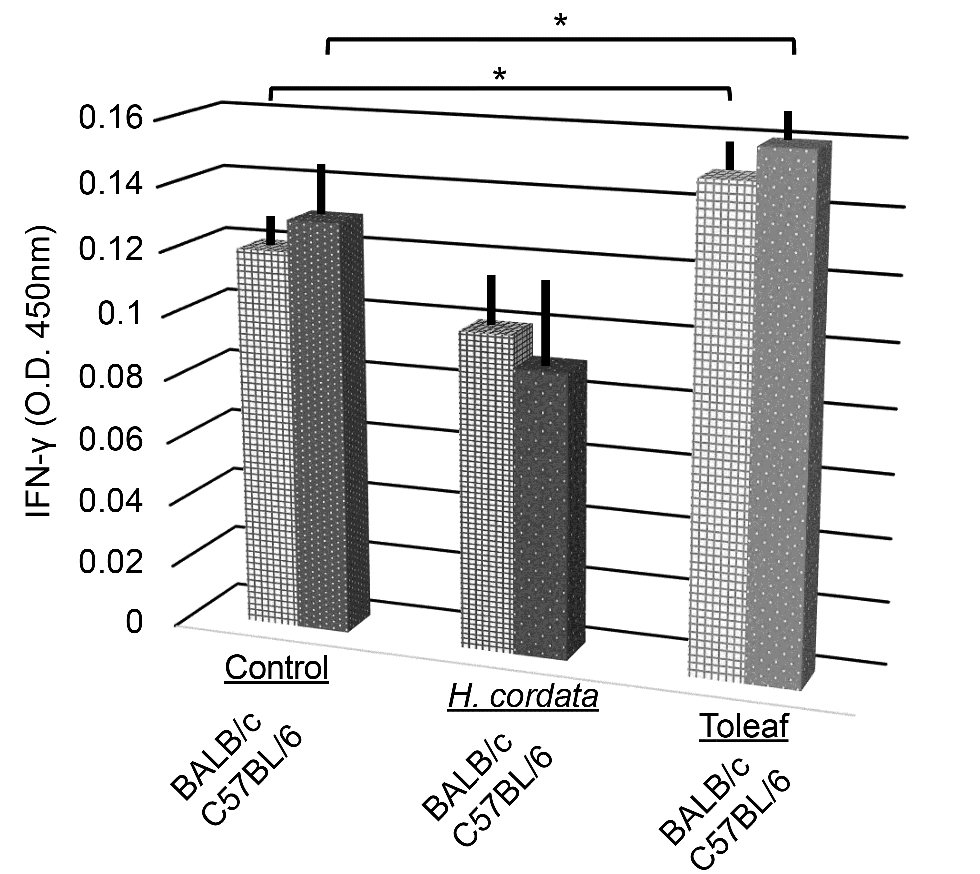 Figure 2. Mouse splenic cell interferon (IFN)-γ secretory capacity assessment using ELISA. Explant mouse splenic cells treated with medicinal plant extracts were incubated for 16 h. IFN-γ release into the supernatant was assessed using ELISA (as mean optical density (O.D.)). * p < 0.01 compared with the control.
Figure 2. Mouse splenic cell interferon (IFN)-γ secretory capacity assessment using ELISA. Explant mouse splenic cells treated with medicinal plant extracts were incubated for 16 h. IFN-γ release into the supernatant was assessed using ELISA (as mean optical density (O.D.)). * p < 0.01 compared with the control.
Upon initial antigen stimulation, Th1 cells produce IFN-γ and IL-2, whereas Th2 cells produce IL-4, among other substances [20,21]. Therefore, to more precisely differentiate Toleaf extract-induced immunological response role in splenic cells, we measured several cytokine (IL-2, IL-4, and IL-12) mRNA expression levels using quantitative RT-PCR. Toleaf extract-treated C57BL/6 splenic cells exhibited significant increases in IL-2, IL-4, and IL-12 mRNA levels (Figure 3). However, H. cordata extract induced IL-2 levels, but suppressed IL-4 levels. BALB/c splenic cells exhibited similar trends.
Thus, Toleaf, but not H. cordata, extract promotes IL-2, IL-4, and IL-12 expression in unstimulated splenic cells. IL-2 is a Th1-type cytokine also involved in cell multiplication, whereas IL-4 is Th2-type cytokine and IL-12 production is a marker of macrophage and dendritic cell activation. This cytokine upregulation produced by Th1 and Th2 cells [20,21] suggests that Toleaf extract activates the adaptive immune system.
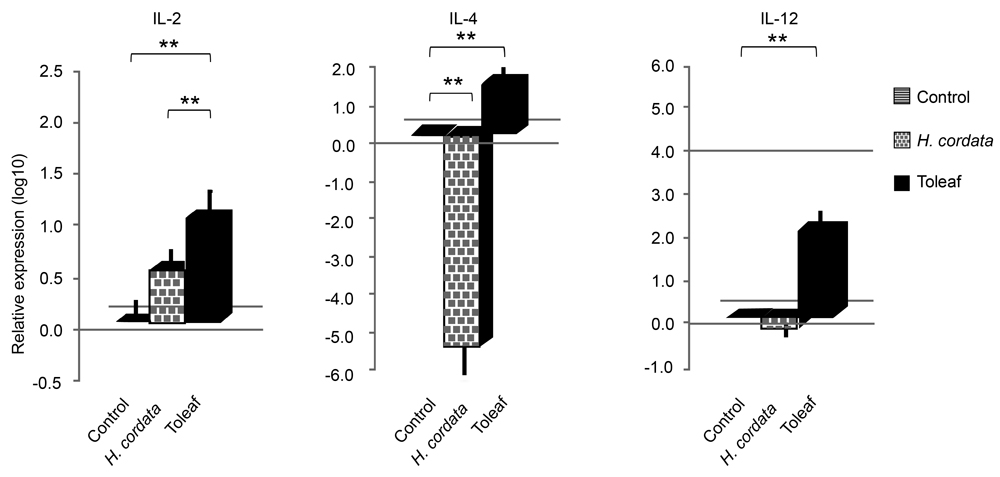 Figure 3. Interleukin (IL)-2, IL-4, and IL-12 mRNA expression in mouse splenic cells. Explant mouse splenic cells treated with Toleaf/H. cordata extracts were incubated for 16 h. IL-2, IL-4, and IL-12 mRNA expression was analyzed by reverse-transcription polymerase chain reaction. Relative expression levels are shown as log10 ratios normalized to the control (untreated cells). ** p < 0.001.
Figure 3. Interleukin (IL)-2, IL-4, and IL-12 mRNA expression in mouse splenic cells. Explant mouse splenic cells treated with Toleaf/H. cordata extracts were incubated for 16 h. IL-2, IL-4, and IL-12 mRNA expression was analyzed by reverse-transcription polymerase chain reaction. Relative expression levels are shown as log10 ratios normalized to the control (untreated cells). ** p < 0.001.
To confirm this hypothesis, we examined murine splenic CD4+ T-cells using flow cytometry. Th1 cells can be characterized by IFN-γ and TNF-α secretion and antigen-presenting cell activation. Th2 cells favor a predominantly humoral response, with Th2 cell development promoted by IL-4. Toleaf- and H. cordata-treated T-cell populations comprised approximately 20% IFN-γ-producing T-cells (Figure 4A). However, the Toleaf-treated T-cell population contained twice as many IL-4-producing T-cells than H. cordata-treated population (Figure 4B). These findings indicate that Toleaf treatment induces Th1 and Th2 T-cell differentiation, whereas H. cordata only induces Th1 cell formation. IFN-γ- to IL-4-producing T-cell ratio, reflecting the Th2/Th1 ratio, is depicted Figure 4C. H. cordata extract treatment resulted in a higher ratio than Toleaf extract treatment.
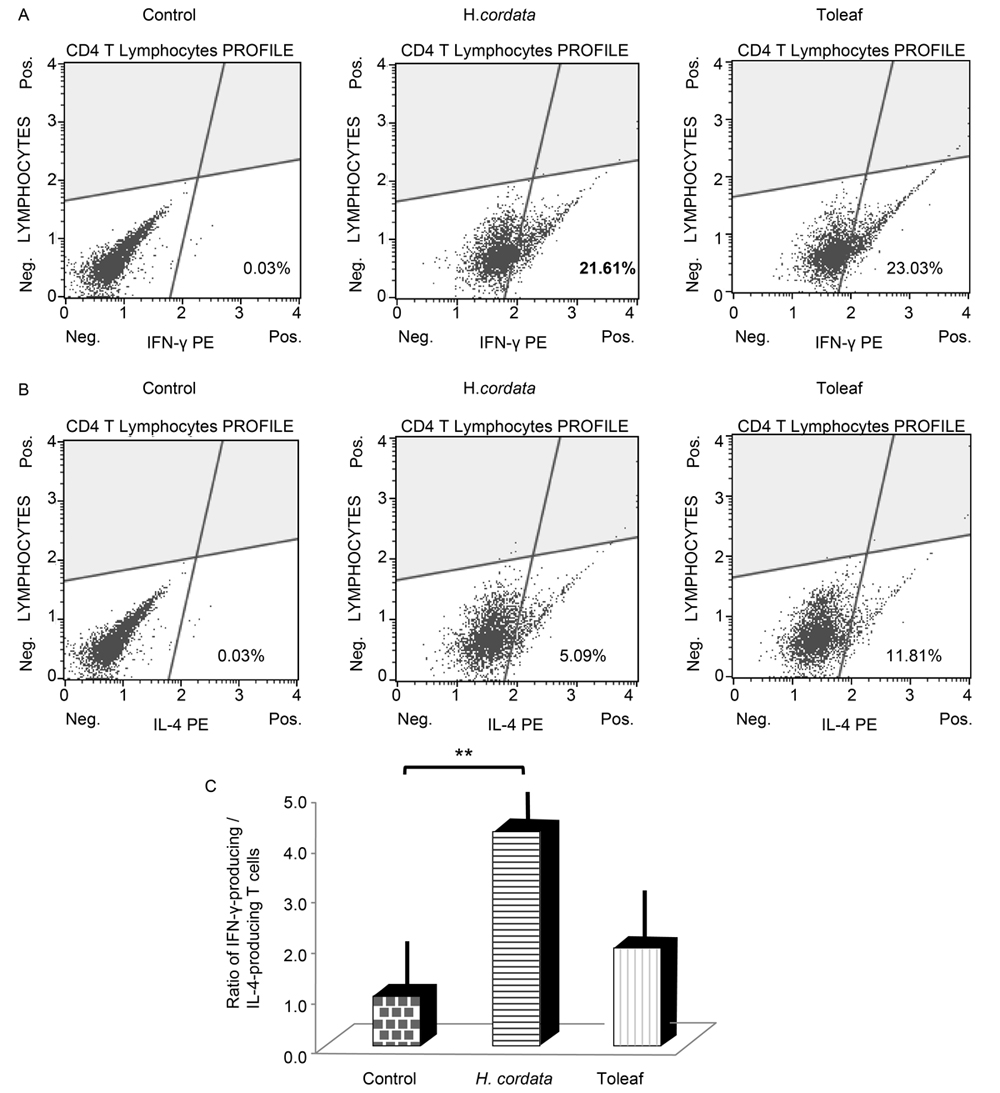 Figure 4. Effects of Toleaf and H. cordata extracts on the adaptive immune system. CD4+ T-cells were isolated from mouse splenic cell suspension by MACS. Isolated T-cells were treated with Toleaf/H. cordata extracts for 7 days. Flow cytometry was used for quantifying (A) interferon (IFN)-γ- and (B) interleukin (IL)-4-producing T-cell populations. Control indicates untreated cells incubated with PE monoclonal rat anti-mouse antibodies targeting IFN-γ/IL-4 (total CD4+ T-cells). (C) Ratio of IFN-γ- to IL-4-producing T-cells indicates the balance between Th1 and Th2 activation. ** p < 0.001.
Figure 4. Effects of Toleaf and H. cordata extracts on the adaptive immune system. CD4+ T-cells were isolated from mouse splenic cell suspension by MACS. Isolated T-cells were treated with Toleaf/H. cordata extracts for 7 days. Flow cytometry was used for quantifying (A) interferon (IFN)-γ- and (B) interleukin (IL)-4-producing T-cell populations. Control indicates untreated cells incubated with PE monoclonal rat anti-mouse antibodies targeting IFN-γ/IL-4 (total CD4+ T-cells). (C) Ratio of IFN-γ- to IL-4-producing T-cells indicates the balance between Th1 and Th2 activation. ** p < 0.001.
We evaluated macrophage phagocytic activity using microspheres for determining whether these medicinal plant extracts affected the innate immune system. Phagocytosis presence indicates isolated murine peritoneal macrophage activation. No remarkable differences in macrophage activity were observed between medicinal plant extract-treated samples and controls (Figure 5). Thus, Toleaf-activated CD4+ Th1 and Th2 cells may mediate its anti-cancer effects.
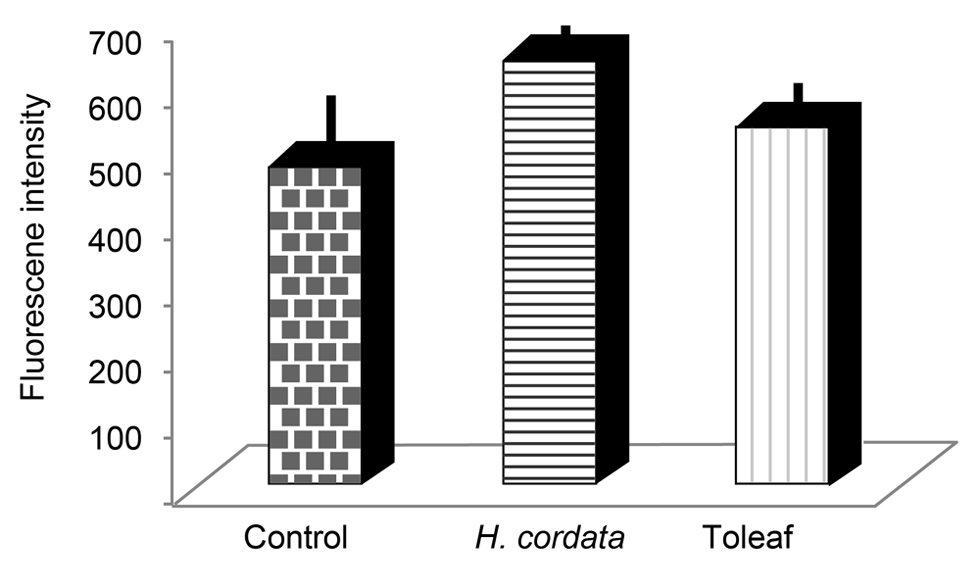 Figure 5. Effects of Toleaf and H. cordata extracts on the innate immune system. Innate immune system activity was examined by macrophage phagocytic activity using microspheres. Macrophages were isolated from mouse intraperitoneal cavities; isolated cells were treated with Toleaf/H. cordata extracts for 1 h and then incubated with microspheres. The fluorescence intensity of microspheres was analyzed via fluorescence microscopy. No statistically significant differences were observed.
Figure 5. Effects of Toleaf and H. cordata extracts on the innate immune system. Innate immune system activity was examined by macrophage phagocytic activity using microspheres. Macrophages were isolated from mouse intraperitoneal cavities; isolated cells were treated with Toleaf/H. cordata extracts for 1 h and then incubated with microspheres. The fluorescence intensity of microspheres was analyzed via fluorescence microscopy. No statistically significant differences were observed.
To confirm this hypothesis and to determine the T lymphocyte activation and Toleaf’s anti-cancer effect relationship, we co-cultured medicinal plant extract-treated isolated T lymphocytes with three cancer cell lines and evaluated their viability. These cell lines represented three cancer types (solid carcinoma, lymphocytic cancer, and epidermal carcinoma), thus allowing to evaluate whether cancer type affects anti-cancer activity.
We first examined direct effects of Toleaf and H. cordata extract on the cancer cell lines. H. cordata extract significantly decreased CT-26 cell viability relative to control- and Toleaf-treated cell viability, whereas Toleaf failed to exert any effects on any of the tested cell lines (Figure 6A). However, Toleaf/H. cordata extract-treated T lymphocytes significantly decreased CT-26 and EL-4 cell viability relative to control viability; Toleaf-treated T lymphocytes exhibited a more pronounced anti-cancer effect (Figure 6B). Only Toleaf-treated CD4+ T lymphocytes had an anti-cancer effect on B-16 cells.
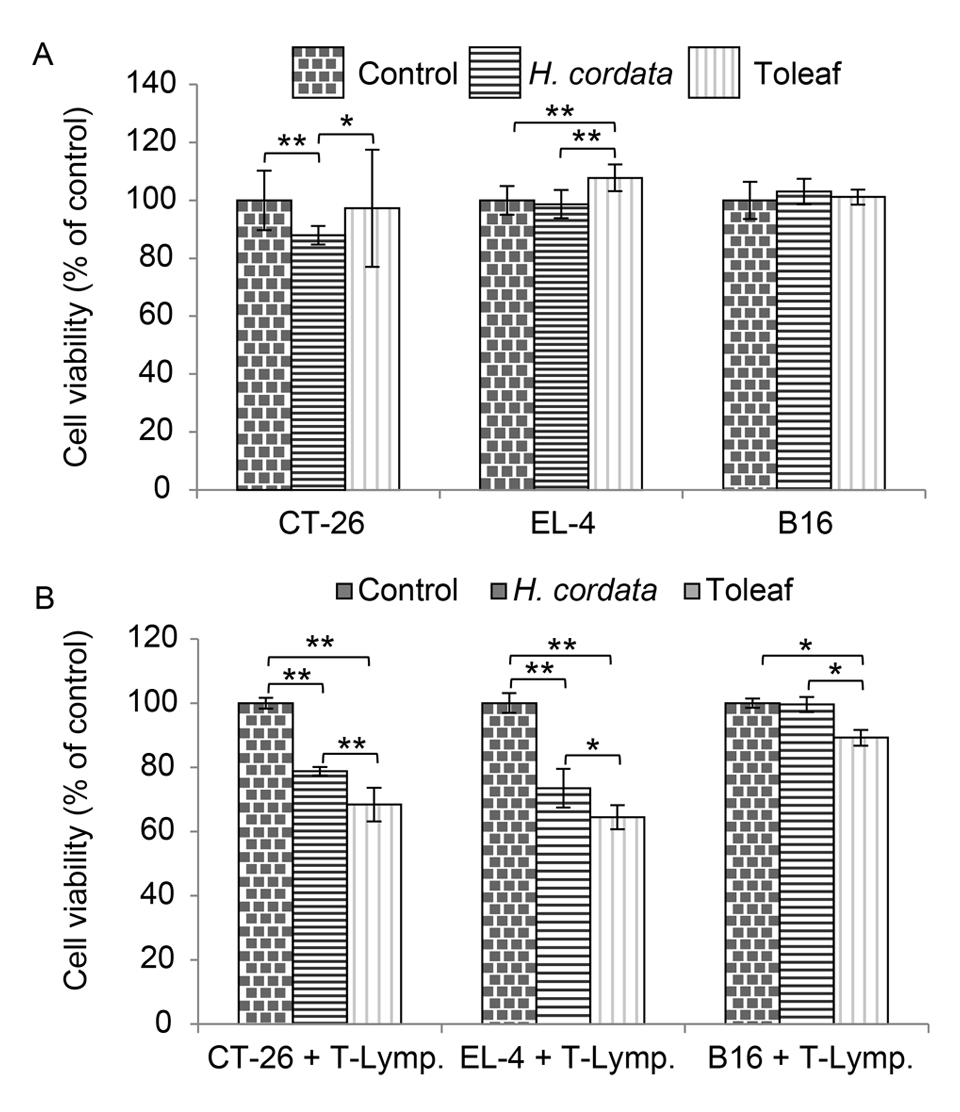 Figure 6. Evaluation of Toleaf and H. cordata extract effects on cancer cell viability. (A) CT-26, EL-4, and B16 cancer cells were treated with Toleaf/H. cordata extract and then incubated for 7 days. (B) The cells were co-incubated for 7 days with isolated T lymphocytes previously treated with Toleaf/H. cordata extract for 7 days. Cell viability was assessed using the lactate dehydrogenase release assay. Data are presented as a percentage of corresponding control values (n = 8). * p < 0.01, ** p < 0.001 (as determined using one-way analysis of variance with Tukey’s post hoc test).
Figure 6. Evaluation of Toleaf and H. cordata extract effects on cancer cell viability. (A) CT-26, EL-4, and B16 cancer cells were treated with Toleaf/H. cordata extract and then incubated for 7 days. (B) The cells were co-incubated for 7 days with isolated T lymphocytes previously treated with Toleaf/H. cordata extract for 7 days. Cell viability was assessed using the lactate dehydrogenase release assay. Data are presented as a percentage of corresponding control values (n = 8). * p < 0.01, ** p < 0.001 (as determined using one-way analysis of variance with Tukey’s post hoc test).
Neither Toleaf nor H. cordata were found to be effective in directly killing cancer cells. On the other hand, the effect of killing cancer cells through lymphocytes was observed, and the effect was stronger with Toleaf.
Therefore, we propose that anti-cancer effects of Toleaf are mediated by CD4+ T lymphocyte activation [6]. Although H. cordata could directly reduce CT-26 cell viability, Toleaf-mediated CD4+ T lymphocyte cytotoxic response was more potent for all three cancer cell lines, possibly indicating differences in mechanisms of action between Toleaf and H. cordata extract.
Effects of Toleaf treatment suggest a relationship between cancer cell killing and CD4+ Th cell (Th1 and Th2) activation. In cancer immunity, several CD4+ T-cell subsets (Th1, Th1, T regulatory, Th17 Th22, and follicular Th) have been described [22]. Here it appeared that CD4+ Th cell activation was more important than increasing the Th2/Th1 ratio.
We examined the relationship between the immune system and anti-cancer effects of Toleaf; Toleaf did not directly induce cancer cell death or activate macrophages. Instead, it impeded cancer cell viability by activating CD4+ Th cells.
KT, KD, TM and KT designed the study. KT and KD performed the experiments. KT, and KD analyzed the data. KT, KD, TM and KT contributed to scientific discussions. KT and KD wrote the paper with input from all authors.
The authors declare no conflicts of interest.
These studies were supported by GHM Co., Ltd.
We would like to thank Baiosu Co., Ltd. and Enago (www.enago.jp) for English language review of the manuscript.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
Takanashi K, Dan K, Matsuoka T, Torii K. The Cancer Cell Killing Effects of Gynura procumbens, Toleaf Are Associated with CD4+ T Lymphocyte Activation. Pharm Front. 2019;1:e190004. https://doi.org/10.20900/pf20190004

Copyright © 2020 Hapres Co., Ltd. Privacy Policy | Terms and Conditions