Location: Home >> Detail
TOTAL VIEWS
Immunometabolism. 2021;3(2):e210015. https://doi.org/10.20900/immunometab20210015
Laboratory of Biochemistry, Department of Medicine, Democritus University of Thrace, Alexandroupolis 68100, Greece
* Correspondence: Charalampos Papadopoulos.
Erythrocytes (red blood cells) interact with both the immune and the metabolic systems. However, emerging data indicate that they can also function as mediators in immunometabolic interactions. High-fat diet results in increased erythrocyte cholesterol, externalized phosphatidylserine, bound MCP1, myeloperoxidase and the generation of reactive oxygen species. As a result, a pro-inflammatory effect of the erythrocyte ensues subsequently triggering macrophage inflammation, chemotaxis, erythrophagocytosis and endothelial activation. Furthermore, as a consequence of metabolic syndrome or obesity, important immunoregulatory molecules of erythrocytes, such as CD47, Glycophorin A and microvesicles are affected. Finally, inflammation-induced lipid remodelling of erythrocytes possibly partakes in a positive feedback loop with inflammation. These studies strongly indicate that erythrocytes contribute to the immunometabolic cross-talk, mainly by linking the systemic metabolic status with innate immunity and inflammation. Further exploration of the implicated mechanisms could lead to potent therapeutic targets for metaflammation-related diseases.
ASMase, acid sphingomyelinase; DARC, duffy antigen receptor for chemokines; GYPA, glycophorin A; HDL, high-density lipoprotein; MCP1, monocyte chemoattractant protein 1; MGF-E8, milk fat globule-EGF factor 8 protein; MPO, myeloperoxidase; PSer, phosphatidylserine, RBC, red blood cell; ROS, reactive oxygen species; SM, sphingomyelin; TNF-α, tumor necrosis factor α
Erythrocytes (red blood cells) interact with both the systemic metabolism and the immune function [1]. Through various surface molecules, red blood cells scavenge chemokines, mitochondrial DNA and regulate the functional phenotypes of dendritic cells, T cells, neutrophils and monocytes. In addition, they are important mediators of reverse cholesterol transport and a source of Damage-Associated Molecular Patterns, bioactive lipids and cytokines in the blood [1]. For instance, they are the main source of an important immunoregulatory lipid, that is sphingosine 1-phosphate [2] that is produced in response to various stimuli [3,4]. These characteristics possibly imply that red blood cells could also mediate interactions between the metabolic and the immune systems, an intersected network known as immunometabolism [5]. The contribution of erythrocytes in the systemic metabolism, the immune function, and the production of potent immunoregulatory metabolites has already been described [1,6]. However, a potential direct connection of immunometabolism through erythrocytes has not been taken into account. In this perspective, we attempt to elucidate the fact that red blood cells are important cellular mediators of immunometabolic signaling, and postulate novel mechanisms (Figure 1). This is of particular significance, since the role of red blood cells is often overlooked in studies investigating immunometabolism.
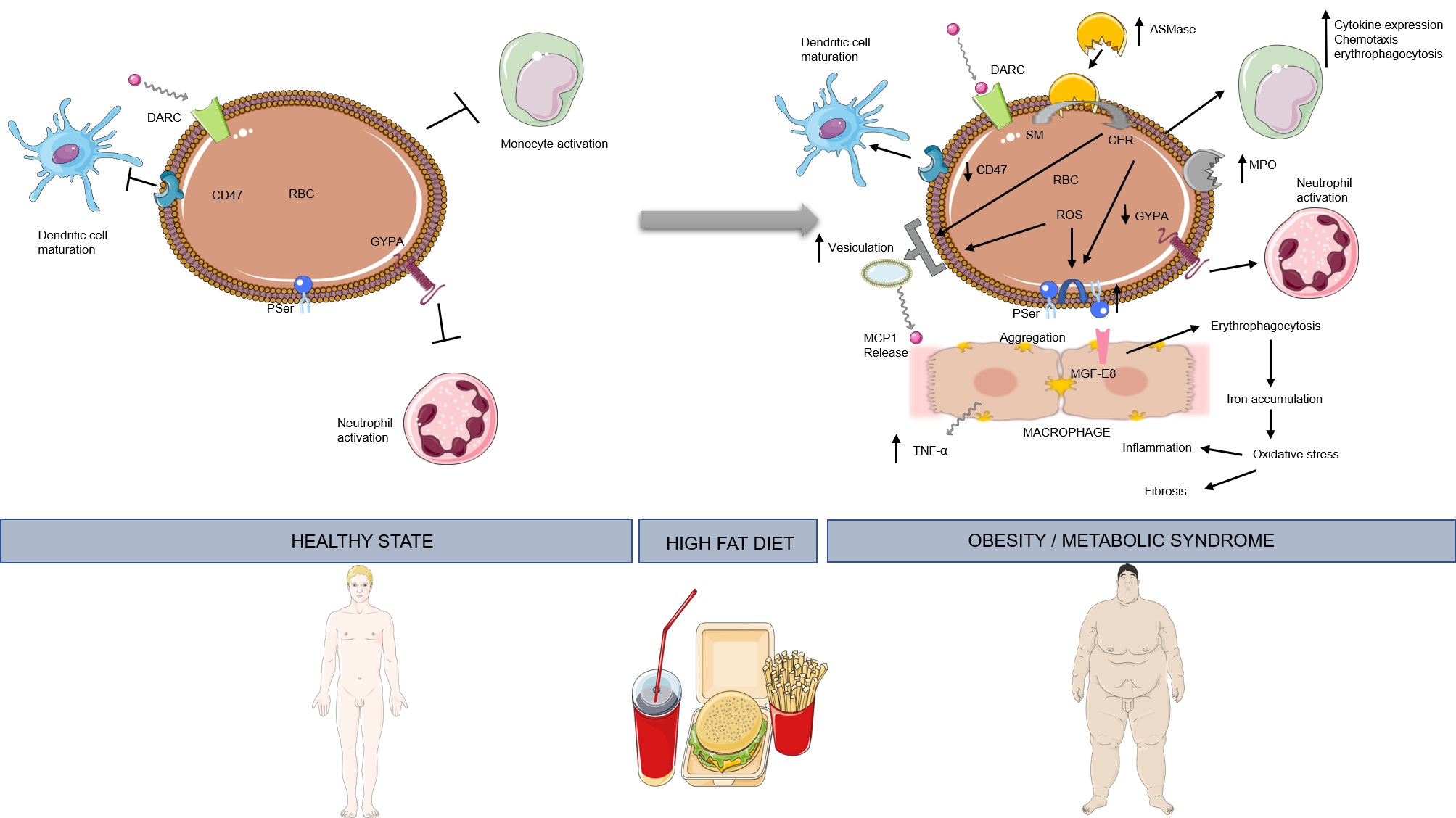 Figure 1. High-fat diet induces a transition to the immunoregulatory function of erythrocytes, which then act as bidirectional mediators between immune function and metabolism. This figure was created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License; https://smart.servier.com.
Figure 1. High-fat diet induces a transition to the immunoregulatory function of erythrocytes, which then act as bidirectional mediators between immune function and metabolism. This figure was created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License; https://smart.servier.com.
The most substantial study regarding the role of red blood cells in connecting systemic metabolism with immune function comes from Unruh et al. [7]. In their study, erythrocytes of mice fed a high-fat diet for 12 weeks, ultimately came up with high content of membrane cholesterol, externalized phosphatidylserine, high levels of markers of reactive oxygen species (ROS) and bound Monocyte Chemoattractant Protein 1 (MCP1). In addition, erythrocytes of these animals induced macrophage chemotaxis in ex vivo experiments. This effect was less profound in high-fat diet-fed mice lacking Duffy Antigen Receptor for Chemokines (DARC). Thus, those findings could be attributed to the release of MCP1 by DARC. Unruh et al. [7], also reported endothelial activation induced by erythrocytes of high-fat diet animals. Heme and/or hemoglobin release could possibly have been the cause of this effect, as these agents are known to induce the expression of adhesion molecules and bring about cytoskeleton organization in endothelial cells [8,9]. Furthermore, red blood cells from high-fat diet mice augmented erythrophagocytosis by splenic macrophages, an effect accompanied by increased transcription levels of pro-inflammatory chemokines [7]. Similar results have been reported by Otogawa et al. [10]. In that study, high-fat diet-induced steatohepatitis triggered erythrocyte phosphatidylserine exposure, resulting in erythrocyte accumulation in the liver through phosphatidylserine and MGF-E8 interaction, erythrophagocytosis by Kupffer cells, and augmentation of inflammation and fibrosis. This latter effect was linked to increased iron accumulation in the Kupffer cells. However, based on the results of Unruh et al. [7], cholesterol accumulation could also contribute to this effect [11].
Studies in humans further unveil the pro-inflammatory role of red blood cells in the context of abnormal systemic metabolism. Benson et al. [12], showed that consumption of a high-fat meal by healthy humans, increased erythrocyte ROS and erythrocyte bound myeloperoxidase (MPO). This correlated with oxidation of high-density lipoprotein [HDL]. In obese individuals, increased erythrocyte phosphatidylserine exposure has been reported [13], a mechanism previously described to enhance hepatic inflammation and fibrosis. In addition, obesity results in loss of CD47 from erythrocyte membranes [14]. Through this molecule red blood cells can regulate the maturation of and the cytokine profile released from dendritic cells [15,16]. Thus, the relation between obesity and erythrocyte CD47 certainly merits further exploration. Furthermore, in patients with metabolic syndrome, erythrocytes present lower Glycophorin A levels [17] and increased production of extracellular vesicles [18]. Since Glycophorin A regulates neutrophil activation [19], and erythrocyte-derived microvesicles affect monocyte activation [20,21] we propose that investigation of their active role in the sub-clinical inflammation observed during metabolic syndrome and obesity could unveil novel therapeutic targets.
More recently, a study by Hazegh et al. [22] provided important cues for the molecular basis of obesity-induced erythrocyte metabolic changes. In their study, they found that obesity of blood transfusion donors was associated with storage, osmotic and oxidative hemolysis. Furthermore, blood donors with high BMI (44.1 ± 5.1 kg/m2) had increased erythrocyte lysophosphatidylinositol, lysophosphatidylserine, short-chain fatty acids and oxidative markers, Changes in the amino acid metabolism were also observed, with arginine, tryptophane and kynurenine (oxidized product of tryptophane), being higher in obese individuals. Additional investigation in mice, showed that donor obesity correlated with lower percentage of post-transfusion recovery. Hence, the role of the abnormal red blood cell metabolome should be investigated with regards to immune function.
The role of red blood cells in immunometabolism could be applicable not only to inflammation and fibrosis, but to immunothrombosis too. Externalized phosphatidylserine of erythrocytes binds pro-thrombinase and induces thrombin formation [23]. Exposure of phosphatidylserine in erythrocytes, and subsequent thrombin activation is also triggered by extracellular histones [24] that are important constituents of immunothrombosis. However, to what extent obesity- and immunothrombosis-induced phosphatidylserine exposure have an additive effect certainly needs further investigation.
Inflammation-induced lipid metabolism in erythrocytes could amplify the immune reaction to sterile inflammation. Increased serum sphingomyelinase levels induced by inflammation trigger ceramide formation, which can then induce phosphatidylserine exposure and microvesicle release from erythrocytes [25]. Interestingly, our unpublished results show an inverse correlation between sphingomyelin content and CCL2 release from red blood cells, in the context of non-alcoholic fatty liver disease. Conditioned-media from these erythrocytes provoked an increased release of TNF-α by RAW 264.7 macrophages. This latter function could be attributed to MCP1 [26]. In addition, MCP1 release by erythrocytes could be attributed to microvesicle release [27], which are formed by sphingomyelin hydrolysis [25]. Thus, a pathway comprised of sphingomyelin hydrolysis-microvesicle release-MCP1 release, similar to that described for glial cells and IL-1β [28], is possible for the case of red blood cells, but merits further exploration.
Red blood cells participate in immunometabolism. Their immunoregulatory molecules, such as CD47, Glycophorin A, phosphatidylserine, DARC, membrane bound MPO and the positive feedback loop between inflammation and erythrocyte lipid metabolism, should be revisited in the future as potent therapeutic targets of immunometabolic diseases, such as atherosclerosis, metabolic fatty liver disease and obesity.
The authors declare that they have no conflicts of interest.
The research work was supported by the Hellenic Foundation for Research and Innovation [HFRI] under the HFRI PhD Fellowship grant (Fellowship Number: 1343).
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
Papadopoulos C, Tentes I, Anagnostopoulos K. Erythrocytes Contribute to the Immunometabolic Cross-Talk. 2021;3(2):e210015. https://doi.org/10.20900/immunometab20210015

Copyright © 2021 Hapres Co., Ltd. Privacy Policy | Terms and Conditions