Location: Home >> Detail
TOTAL VIEWS
Immunometabolism. 2021;3(4):e210027. https://doi.org/10.20900/immunometab20210027
Laboratory of Biochemistry, Department of Medicine, Democritus University of Thrace, Alexandoupolis 68100, Greece
* Correspondence: Charalampos Papadopoulos, Tel.: +30-695-172-9401.
In this commentary we discuss the novel findings of Gerner et al. In their study, it was found that red blood cells inhibit T cell activation through scavenging of reactive oxygen species. We propose a novel immunometabolic pathway, based on previous studies which showed that hyperglycemia abolished the antioxidant function of red blood cells.
GPX, glutathione peroxidase; GSH, glutathione; GSH R, glutathione reductase; GSSG, glutathione disulfide; NADPH, nicotinamide adenosine dinucleotide phosphate; PPP, Pentose phosphate pathway; RBC, red blood cell; ROS, reactive oxygen species
Red blood cells (erythrocytes) contribute to systemic metabolism as well as immune function [1]. We recently provided a novel viewpoint for the role of red blood cells in immunometabolism [2]. In that viewpoint, red blood cell composition of immunomodulatory molecules is altered as a result of dramatic changes in systemic metabolism. This further amplifies the immune response. In view of the role of red blood cells in immunometabolism, erythrocyte dysfunction has been studied in relation to immunometabolic diseases, such as non-alcoholic fatty liver disease [3], atheromatosis [4] and lipotoxicity [5] in general.
Recently, the effect of red blood cells on T cell function was extensively investigated by Gerner et al. [6]. In their study they proved that packed red blood cells can inhibit the activation of CD4+ T lymphocytes. In particular, they found that incubation of erythrocytes with CD4+ T lymphocytes, inhibited T cell proliferation and the expression of IL-2 and cytokines specific of Th1, Th2 and Th17 cells, without promoting differentiation towards T regulatory cells. This inhibitory effect was observed in both naïve and memory T lymphocytes. Interestingly, the suppressive effect of red blood cells on T cells required cell contact. Mechanistically, red blood cells inhibited ROS production. Subsequently, this is linked to reduced activity of important signaling molecules required for T cell activation: mitogen-activated protein kinase, cyclin-dependent kinase 5 and mechanistic target of rapamycin (mTOR) targets.
We believe that the results of Gerner et al., could have major implications for the immunometabolic landscape of hyperglycemia. Morabito et al. [7], have found that in vitro incubation of erythrocytes in presence of high glucose levels results in lower levels of the GSH/GSSG ratio. In the same study, erythrocytes from diabetic individuals also presented lower levels of the GSH/GSSG ratio. The results of that study could be attributed to the formation of advanced glycation end products (AGE) which are sources of ROS. GSH is one of the antioxidant molecules of red blood cells, permitting erythrocytes to function as a ROS scavenger in the circulation, mainly through the action of glutathione peroxidase (GPX) [8]. In this perspective, high blood glucose levels, induce the formation of advanced glycation end products in hemoglobin and membrane molecules [9] of red blood cells. AGE comprise a major source of reactive oxygen species, and, thus consume erythrocyte GSH.
Alternatively, low levels of the GSH/GSSG ratio could be attributed to lower de novo synthesis of GSH. Palomina-Schatzlein et al. [10], have found, in type 2 diabetes patients, major alterations in the levels of aspartate and glutamine, amino acids that contribute to the formation of GSH. However, it has been previously shown that the cause for decreased GSH formation in type 2 diabetes is not reduced synthesis of GSH, but the inability to reduce GSSG to GSH [11].
Hence, we propose a novel immunometabolic pathway, where high glucose levels during hyperglycemia could lead to lower GSH levels, through consumption of GSH by hemoglobin- and membrane- AGE, thus disrupting the balance between GSSG formation and reduction. Consequently, red blood cells would lose their capacity to scavenge ROS originating from T lymphocytes (Figure 1). As a result, T cells could be activated, leading to the intensification of high glucose-related pathologies [12].
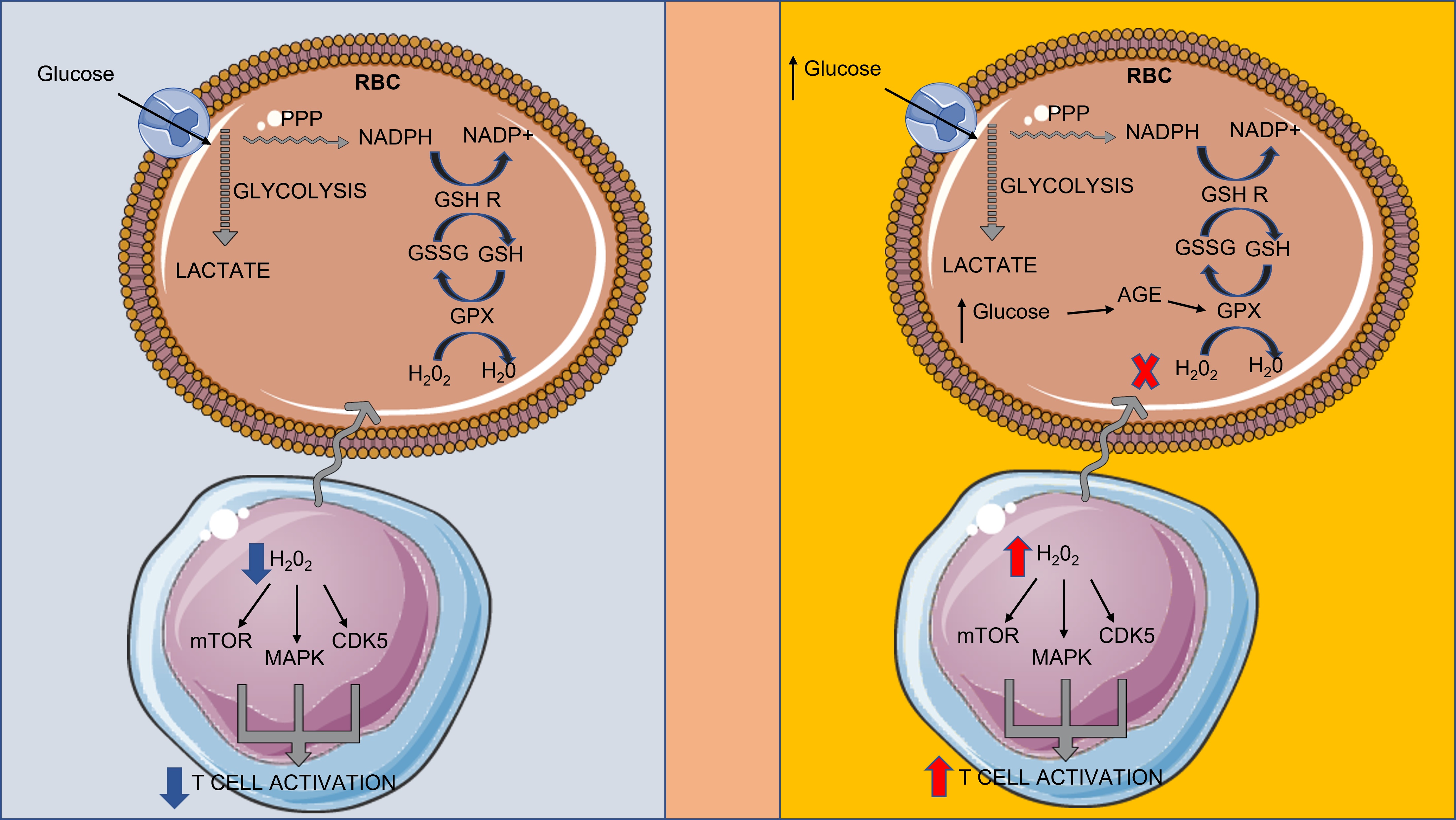 Figure 1. Hyperglycemia induces the formation of advanced glycation end products, which consume GSH, interfering with RBC-mediated suppression of T cell activation. CDK5: cyclin-dependent kinase 5; GPX: glutathione peroxidase; GSH: glutathione; GSH R: glutathione reductase; GSSG: glutathione disulfide; MAPK: mitogen-activated protein kinase; and mTOR: mechanistic target of rapamycin targets NADPH: nicotinamide adenosine dinucleotide phosphate; PPP: Pentose phosphate pathway; RBC: red blood cell; ROS: reactive oxygen species.
Figure 1. Hyperglycemia induces the formation of advanced glycation end products, which consume GSH, interfering with RBC-mediated suppression of T cell activation. CDK5: cyclin-dependent kinase 5; GPX: glutathione peroxidase; GSH: glutathione; GSH R: glutathione reductase; GSSG: glutathione disulfide; MAPK: mitogen-activated protein kinase; and mTOR: mechanistic target of rapamycin targets NADPH: nicotinamide adenosine dinucleotide phosphate; PPP: Pentose phosphate pathway; RBC: red blood cell; ROS: reactive oxygen species.
The authors declare that they have no conflicts of interest.
The research work was supported by the Hellenic Foundation for Research and Innovation [HFRI] under the HFRI PhD Fellowship grant (Fellowship Number: 1343).
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
Papadopoulos C, Anagnostopoulos K, Tentes I. An Immunometabolic Pathway for Hyperglycemia?—Commentary on: Packed Red Blood Cells Inhibit T-Cell Activation via ROS-Dependent Signaling Pathways. Immunometabolism. 2021;3(4):e210027. https://doi.org/10.20900/immunometab20210027

Copyright © 2021 Hapres Co., Ltd. Privacy Policy | Terms and Conditions