Location:Home >> Detail
TOTAL VIEWS
Med One. 2018; 3: e180001. https://doi.org/10.20900/mo.20180001
1 Department of Pharmacy, International Islamic University Chittagong, Chittagong 4318, Bangladesh
2 Department of Biochemistry & Molecular Biology, University of Chittagong, Chittagong 4331, Bangladesh
3 Department of Pharmacy, Jessore University of Science and Technology, Jessore 7408, Bangladesh
a These authors are joint first author on this work
*Corresponding Author: A.S.M. Ali Reza, Assistant Professor, Department of Pharmacy, International Islamic University Chittagong, Kumira, Sitakunda, Chittagong-4318, Bangladesh. Tel: +88-031-610085, Fax: +88-031-610307.
Background: The study was designed to investigate the phytochemical, analgesic, anti-arthritic, thrombolytic and cytotoxic evaluation of the Begonia roxburghii (Miq.) DC leaves extract (nHBR) using both in vitro and in vivo methods.
Methods: The acetic acid induced writhing test and formalin induced pain test was used to perform anti-nociceptive activity on mice. In vitro anti-arthritic activity was assessed using protein denaturation method. The clot lysis activity was considered to evaluate thrombolytic potentials of the plant extract. The cytotoxic study was performed using brine shrimp larvae.
Results: The phytochemical study has shown the abundance of flavonoids, alkaloids, glycosides, tannins, saponins and reducing sugars in the plant extract. In acetic acid-induced writhing test, the extract at different doses (100-400 mg/kg) reduced the number of writhing by 26.42 ± 1.13, 43.64 ± 2.50 and 55.42 ± 1.83 % respectively. The nHBR inhibited the licking response in both the early phase (56.52 ± 2.55 %) and the late phase (62.52 ± 2.57 %) in the formalin induced pain test. Moreover, the results revealed concentration dependent anti-arthritic effect in protein denaturation method with maximum inhibition 69.61 ± 2.15 % at doses 1000 μg/ml. The nHBR also showed (47.77 ± 1.80 %) clot lytic activity and cytotoxicity with LC50 67.61 μg/ml in the brine shrimp larvae bioassay.
Conclusion: The study demonstrated phytochemical, anti-nociceptive, anti-arthritic, clot lysis and cytotoxic activities of the Begonia roxburghii (Miq.) DC.
Nowadays, medicinal plants are considered as potential sources of new drugs. Scientists are screening medicinal plants to find out novel drugs against different diseases. In Bangladesh, the folk medicinal practitioners use medicinal plants directly as primary health care therapeutics[1, 2]. The folk medication based on skill and plants with a history of traditional use should be tested using modern methods to find out novel therapeutics. Tissue damage is responsible for pain which is associated by unpleasant sensory and emotional experience[3, 4]. Pain is also interfere our daily life activity and enhance absenteeism from work, underemployment and unemployment and results in a massive economical loss to the persons[5]. Non-steroidal anti-inflammatory drugs are recommended for the management of mild to moderate pain, while steroidal and opioid drugs are used to mitigate acute and chronic pains. But several adverse effects of these drugs such as gastrointestinal disturbances, cardio vascular diseases, renal dysfunction, drug tolerance and drug dependence limit their free usages[6, 7]. Rheumatoid arthritis (RA) is a chronic inflammatory disorder characterized by ankle joint inflammation and formation of synovium, pannus leades to changes the morphology of the joint[8]. Although for the treatment of RA numerous drugs have been developed and used such as Nimesulide, Nimesulide, Abatacept but they are too expensive and have adverse effects [9]. The streptokinase, urokinase and tissue plasminogen activators are thrombolytic medication used for the emergency management of stroke and heart attack. But uses of this drug is linked with high risk of bleeding disorder and allergic problems[10, 11]. Moreover, these drugs are contraindicated for the patients who have a history of nervous lesions, bleeding disorder and low blood pressure[10]. Therefore, the herbal medicines coup up the conventional medication due to their pharmacological activities, money making capability and less or no side effects in different cultures throughout the world[12].
Begonia roxburghii (Miq.) DC. (B. roxburghii) belongs to Begoniaceae family. It is an annual herb and is distributed widely in the in shady moist places of India, Nepal, China and Bangladesh. In Bangladesh B. roxburghii is distributed widely in the marginal forests such as Chittagong, Cox's Bazar, Moulvibazar and Sylhet. The leaves and roots of B. roxburghii are widely used in the treatment of tongue abnormalities, diarrhea, dysentery and jaundice[13]. Moreover, fresh leave juice extract is directly consumed for management of diabetes[14]. Hence, the objective of this study was to assess the preliminary phytochemicals, analgesic, anti-arthritic, thrombolytic and cytotoxic properties of B. roxburghii.
Sodium citrate, sodium phosphate, albumin and diclofenac sodium were purchased from Merck (Mumbai, India) through Taj Scientific Ltd. Bangladesh. Commercially available lyophilized Streptokinase (SK) vial (Durakinase, Dongkook Pharma. Co. Ltd, South Korea) was procured from local market. Acetic acid, Vincristine sulfate were purchased from Sigma-Aldrich (Taufkirchen, Germany). Analytical grade n-hexane was procured from local suppliers and distilled to purify for experiments.
2.2 Plant materialsFresh leaves of Begonia roxburghii (Miq.) DC. were collected from the hill track area of Shitakunda, Chittagong, Bangladesh (March 2016) and plant sample was identified by an expert taxonomist Dr. Sheikh Bokhtear Uddin who is working in the Department of Botany of University of Chittagong. A voucher specimen (accession No. 23164) has been preserved in the aforementioned department.
2.3 Preparation of crude extractThe collected leaves were cleaned, shade-dried and finally in a mechanical drier (Ecocell, MMM Group, Germany). The dried samples were powdered (700 g) to extract with n-hexane (1000 ml). The powdered material placed into an amber bottle for a 7-days-exhaustive extraction with occasional stirring and shaking. The extracts were filtered and concentrated under reduced pressure using rotary evaporator (RE200, Bibby sterling Ltd, UK) to have a black-green semisolid of about 28.5 g.
2.4 Experimental animalsSwiss Albino mice weighing 35-40 g of both male and female were collected from International Center for Diarrheal Diseases Research, Bangladesh (ICDDRB) and housed in polypropylene cages under controlled conditions. The animals were exposed to alternative 12:12 h light and dark cycle at an ambient temperature of 26 ± 2°C. Animals were allowed free access to drinking water and pellet diet, collected from ICDDRB, Dhaka. Mice were acclimatized for seven days in the laboratory environment prior to the study. The set of rules followed for animal experiment were approved by the institutional animal ethics committee, Department of Pharmacy, International Islamic University Chittagong, Bangladesh according to governmental guidelines[15].
2.5 Phytochemical screeningThe qualitative phytochemical screening was performed by standard procedures[16] and the results revealed presence or absence of secondary plant metabolites such as alkaloids, flavonoids, tannins, saponins, phenol, carbohydrate, glycoside.
2.6 Antinociceptive activity 2.6.1 Acetic acid-induced writhing testEither sex of mice (n = 5) weighing 35-40 g was used and divided into 5 groups. Normal control group received normal saline (10 ml/kgbw), reference control group received standard drug diclofenac sodium (10 mg/kgbw) while the rest of the groups were injected intraperitoneally with 100, 200, and 400 mg/kgbw of nHBR. After 30 min of administration, the animals were injected (i.p.) 1 % (v/v) (10 ml/kgbw) acetic acid. After 5 min of acetic acid injection, abdominal constrictions were counted for 10 min and the responses were compared with control group[17, 18]. Antinociceptive activity was calculated as the writhing percentage of inhibition. The percentage of inhibition was calculated using the following ratio:

This biphasic method employed in mice model was assessed as described previously by Hunskaar and Hole[19]. Formalin solution (2.5 %, 20 µl) prepared by 0.9 % saline solution was injected into the sub-plantar region of the right hind paw of mice. Animals were pretreated intraperitoneally with vehicle (0.1 ml/kg saline solution), diclofenac sodium (10 mg/kg) and different doses of extract (100, 200 and 400 mg/kg) 1 h prior to formalin injection. Responses measured for 5 min is considered as first phase and 15-30 min is considered as second phase after formalin injection. First phase and second phase response corresponds to the neurogenic and inflammatory pain responses, respectively. Antinociceptive activity was calculated as the percentage inhibition of licking time.
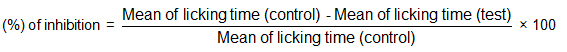
Anti-arthritic activity of the extracts was determined using the protein denaturation method[20]. The constituents of reaction solution were 100 μl nHBR (final concentration 125-1000 μg/ml) and 100 μl of 5 % aqueous bovine serum albumin then the pH was adjusted using glacial acetic acid. The samples were incubated at 37°C for 20 min and then heated to 70°C for 10 min. After incubation the mixture was allowed to cool for 10 min and a turbid solution was found. Then the turbidity was measured at 660 nm. The blank consist of the sample and distilled water. Distilled water was used as the negative control. The positive control was diclofenac sodium. Percentage inhibition was calculated using the formula:
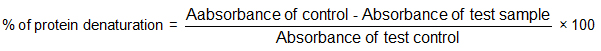
The clot lysis activity were performed as described earlier[21]. A 3 ml venous blood drawn from the healthy volunteers was distributed in nine different pre-weighed sterile microcentrifuge tube (0.5 ml/tube) and incubated at 37°C for 45 min. After clot formation, the developed serum was removed without disturbing the clot and each tube having clot was again weighed to determine the clot weight (clot weight = weight of clot containing tube - weight of tube alone). The plant extracts was added to each microcentrifuge tube separately containing pre-weighed clot. As a positive control, 100 μl of streptokinase (SK) and as a negative non-thrombolytic control, 100 μl of distilled water were separately added to the control tubes. All the tubes were then incubated at 37°C for 90 min and observed for clot lysis. After incubation, fluid released was discarded and tubes were again weighed. Difference obtained in weight taken before and after clot lysis was expressed as percentage of clot lysis. The experiment was repeated with the blood samples of the 10 volunteers without a history of oral contraceptive or anticoagulant therapy since two weeks.
2.9 Brine shrimp cytotoxicity 2.9.1 Assay procedureThe experiment was carried out with the method as reported earlier[22]. The crude nHBR was dissolved in DMSO to obtain a solution of 5 mg/ml which was subjected to serial dilution getting concentrations between 20 to 100 μg/ml. A 5.0 ml of artificial sea water was added to all the test tubes. Then 10 shrimps transported into each vial and incubated for 24 h under light at room temperature (25-29°C) and the survivors were counted with the help of a magnifying glass. Experiments were conducted in a set of three tubes per dose along with vincristine sulfate. The lethal concentration (LC50) that would kill one half of the nauplii was determined from a linear regression equation.
2.9.2 Statistical analysisThe data was analyzed by one-way ANOVA followed by Dunnet’s test to estimate significant differences between the test and control groups with GraphPad Prism Data Editor for Windows, Version 5.0 (GraphPad Software Inc., San Diego, CA). Values were expressed as a mean ± standard error of mean (± SEM). p values (< 0.05 - < 0.01) were considered as statistically significant.
The qualitative phytochemical analysis conducted on nHBR extract revealed the presence of medicinally active different types of secondary metabolites, namely carbohydrates, alkaloids, flavonoids, glycosides, tannins, saponins while absence of steroids and terpenoids (Table 1).
Bioavailability Key: (-) = not present; (+) = present in low concentration; (++) = present in moderately high concentration; (+++) = present in very high concentration.
Data for acetic acid-induced writhing test in mice are shown in Fig.1. The positive control group caused the minimum reduction of the number of writhing (18.05 ± 1.51) and showed the maximum inhibition of 68.33 ± 1.10 % at dose 10 mg/kg. The nHBR was able to inhibit writhing significantly while compared to the control group (p < 0.01). Mention that, the test control group showed number of writhing 26.38 ± 2.15, 33.78 ± 1.19 and 22.30 ± 1.12 at doses 100, 200 and 400 mg/kg respectively. The percentages of inhibition of constrictions were calculated as 26.42 ± 1.13, 43.64 ± 2.50 and 55.42 ± 1.83 % for nHBR of doses of 100, 200 and 400 mg/kg respectively.
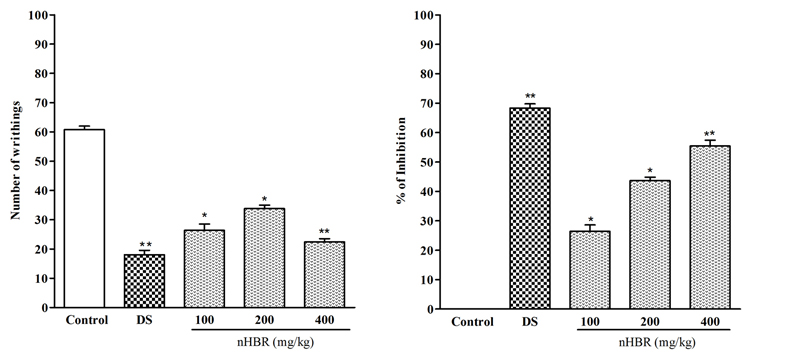 Fig. 1 Effect of n-hexane extract of the Begonia roxburghii and DS (10 mg/kg) on acetic acid induced writhing test.
Fig. 1 Effect of n-hexane extract of the Begonia roxburghii and DS (10 mg/kg) on acetic acid induced writhing test.
Values are mean ± S.E.M. *p < 0.05 and **p < 0.01, significantly different from control; ANOVA followed Dunnett’s test (n = 5, per group). DS: diclofenac sodium, nHBR, Begonia roxburghii leaves extract.
The characteristics biphasic nociceptive responses of nHBR are presented in Fig. 2. The nHBR produced a dose-dependent inhibition of nociception both neurogenic (0-5 min) and inflammatory (15-30 min) phases at the doses of 100, 200 and 400 mg/kg. The maximum inhibition of 62.52 ± 2.57 % was obtained at 400 mg/kg i.p in the late phase of the formalin test. Whereas, in the early phase of licking the highest inhibition was 56.52 ± 2.55 % i.p at 400 mg/kg which is significant compared to the positive control group (73.52 ± 3.25 %) at dose 10 mg/kg.
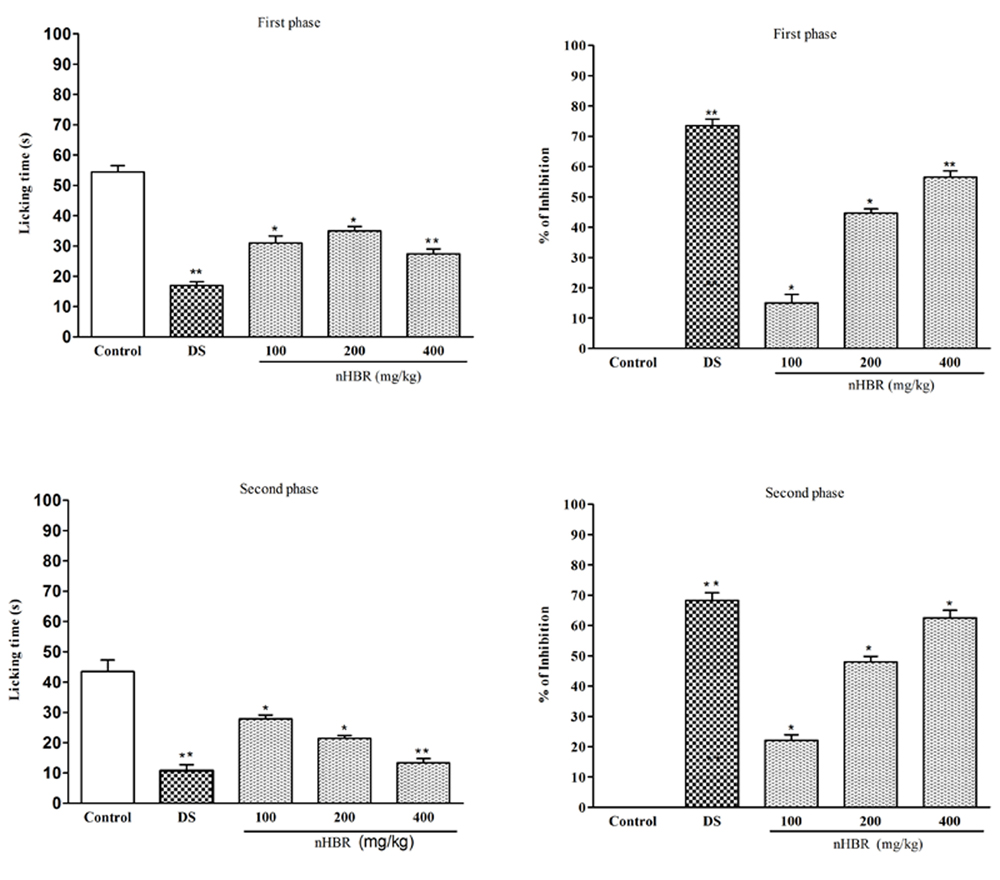 Fig. 2 Effect of n-hexane extract of the Begonia roxburghii and DS (10 mg/kg) on formalin test (first phase and second phase).
Fig. 2 Effect of n-hexane extract of the Begonia roxburghii and DS (10 mg/kg) on formalin test (first phase and second phase).
Values are mean ± S.E.M. *p < 0.05 and **p < 0.01, significantly different from control; ANOVA followed Dunnett’s test (n = 5, per group). DS: diclofenac sodium, nHBR, Begonia roxburghii leaves extract.
The anti-arthritic activity was performed using bovine serum albumin (BSA). The results demonstrated the significant (p < 0.05) inhibition of protein denaturation of nHBR as shown in Fig.3. The anti-arthritic activities were increased with the dose in a noteworthy manner. The crude extracts demonstrated 40.70 ± 2.50, 50.54 ± 3.75, 64.37 ± 1.85 and 69.61 ± 2.15 % inhibition of protein denaturation at 125, 250, 500 and 1000 μg/ml respectively, whereas the reference drug diclofenac sodium showed maximum 83.50 ± 3.25 % inhibition at a dose of 1000 μg/ml.
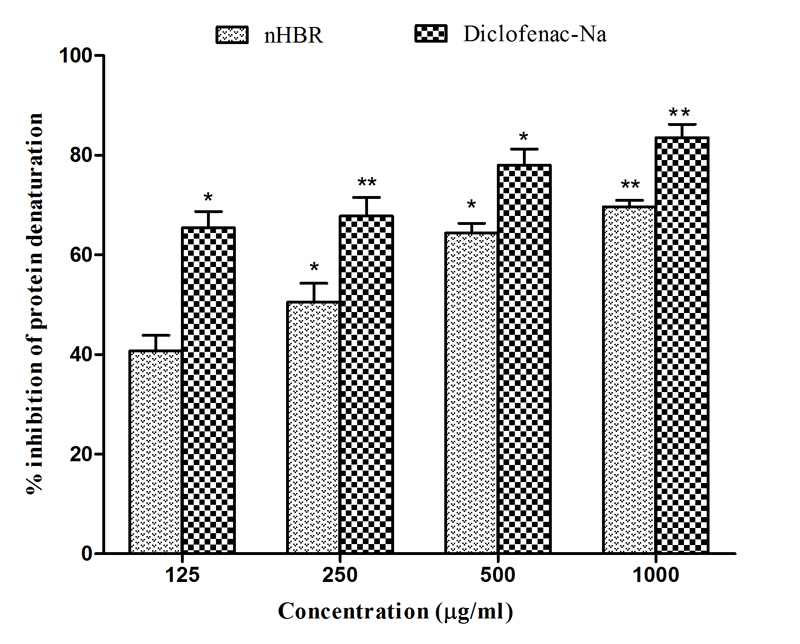 Fig. 3 Effect of n-hexane extract of the Begonia roxburghii against protein denaturation using bovine serum albumin.
Fig. 3 Effect of n-hexane extract of the Begonia roxburghii against protein denaturation using bovine serum albumin.
All the values are expressed as mean ± SEM (n = 3) ** = p < 0.01, * = p < 0.05 vs Diclofenac-Na, nHBR, Begonia roxburghii leaves extract.
The results of the thrombolytic test are shown in Fig. 4. The nHBR had a significant clot lysis effect when after addition of 100 μl SK, a positive control (30,000 I.U.) to the clots along with 90 minutes of incubation at 37°C, provided 73.13 ± 2.80 % clot lysis. On the other hand, sterile distilled water was treated as negative control which showed only 9.66 ± 1.20 %, a negligible clot lysis. Clots when treated with 100 μl of nHBR exhibited 47.77 ± 1.80 % clot lysis comparing with the positive control the mean clot lysis percentage difference was significant (p < 0.05).
Values are expressed as mean ± SEM. p < 0.05, significantly different from control; ANOVA followed by Dunnett’s test. nHBR, Begonia roxburghii leaves extract.
The lethality of the nHBR was determined on a simple zoological organism (Artemia salina). The results from screening of nHBR against Artemia salina larvae are shown in Fig. 5. The degree of lethality was found to be directly proportional to the concentration of test extracts, which provided linearity in the dose-effect relationship and determination of the LC50 value. In this assay, nHBR exhibited an LC50 value of 67.61 μg/mL, which was significantly (p < 0.05) different in reference to positive control vincristine sulfate (50.11 μg/mL), indicating the lower toxicity of the tested extract.
Biological activities of medicinal plants are not only useful for cultural traditions and biodiversity but also for development of new drugs [23]. Medicinal plants are the major resource of bioactive secondary metabolites which is differs from one plants to another plants. The bioactive secondary plant metabolites acts as a part of integrated health care system because of their therapeutic role in prevention of chronic and degenerative diseases[24, 25].
Acetic acid-induced writhing test evaluated the antinociceptive activity of nHBR characterizing through abdominal contractions, movements of the body as a whole and twisting of the dorso-abdominal muscles. In this method, the acetic acid helps to induce the release of inflammatory mediators such as bradykinin and serotonin, which stimulate the nociceptive neurons[26, 27]. The intraperitoneal administration of nHBR remarkably reduced the writhing induced by acetic acid. The formalin test is one of the widely used methods of expressing pain and analgesic mechanism in contrast to mechanical or thermal stimulus methods[28]. In this test the nHBR has shown the ability to affect both the early and late phase inflammatory effects. Mention that, capability of acting both phase implies the involvement of central and peripheral pain relief activity of the extract. The early phase demonstrates as neurogenic pain, an acute response observed immediately after the administration of formalin due to direct stimulation of nociceptive neurons. While the late phase gives a delayed response made by the release of inflammatory mediators especially prostaglandins, histamine, serotoninand bradykinin, and activation ofthe neurons in the dorsal horns of the spinal cord[29]. The early and late phases have used to characterize the analgesic potentials as well as to explain the mechanisms of antinociception. It has been reported that the narcotics or opioid analgesics exert its antinociceptive effects for both phases while the first phase is more sensitive whereas NSAIDs seem to suppress only the second phase[30]. Therefore the administration of nHBR in different doses significantly inhibited the pain response in both phases as confirmed by reduced licking behavior. This biphasic reducing liking behavior is an indicator of neurogenic and inflammatory pain modulators.
The bovine serum albumin (BSA) is used to evaluate anti-arthritis activity of nHBR and BSA is heated and it undergoes denaturation[20]. Moreover, the denaturation of protein is one of the reasons of rheumatoid arthritis. The mechanism of denaturation probably involves alteration of electrostatic, hydrogen, hydrophobic and disulphide bonds [31]. The productions of auto antigen are associated with type-III hypersensitivity reaction and responsible for several diseases such as serum sickness, glomerulonephritis, rheumatoid arthritis and systemic lupus erythematosus[32]. The nHBR showed dose dependent inhibition of protein denaturation. The promising activities of B. roxburghii leave extracts are due to the capability of controlling the production of auto antigen and thereby it inhibits the denaturation of protein.
The development of thrombus is the results of imbalance activity between thrombogenic factors and protective mechanisms, which is accelerated several vascular diseases including stroke, myocardial infarction, deep vein thrombosis, portal vein thrombosis, renal vein thrombosis[33]. In this study, the extract showed significant (p < 0.05) thrombolytic activity comparing with standard drug streptokinase. It has been reported that, presence of secondary plant metabolites like flavonoids might have the capability of clot lysis[34]. The preliminary phytochemical screening of this study showed that the nHBR contain abundance of flavonoids and the presence of plenty of flavonoids might be the one of the causes of thrombolytic activity.
The toxicity of medicinal plant is the prime concern to the practitioners[35] and hence cytotoxic screening was carried out using brine shrimp assay to determine the preliminary toxicity data of the plant extract. Moreover, researchers showed a good correlation (r = 0.85; p < 0.05) between the LC50 of the brine shrimp lethality test and the acute oral toxicity assay in mice[36]. Lagarto showed that, the LC50 < 10 μg/ml (LD50 between 100 and 1000 mg/kg) is considered as the cutoff value of cytotoxicity[36]. Based on the above correlation the B. roxburghii leave showed lower toxicity (LC50 = 67.61 μg/mL) and to be possessed for the production of analgesic, anti-arthritic and thrombolytic drugs.
The results of this study discovered that, the extract significantly showed both peripheral and central analgesic activity in established in vivo models. Then, the results obtained in this study may rationalize the potential anti-arthritic and thrombolytic activity with lower toxicity. The study corroborates traditional claims of the use of this medicinal plant in the management of pain, arthritis and thrombosis. Further work will ascertain the molecular structures and the specific pharmacology of the active principles.
The authors declare that they have no competing interests.
HM, SK and NS carried out the experiments. MT, AA and RK wrote the manuscript. ASMAR and MSN supervised the work and prepared the manuscript. MOR contributed to the manuscript corrections. All authors read and approved the final manuscript.
We are grateful to the Department of Pharmacy, International Islamic University Chittagong, Bangladesh, for providing facilities for this research work.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
Mobarak H, Meah M S, Sikder N, Md. Tareq, Azad A, Khatun R, Nasrin M S, Raihan M O, A.S.M. Ali Reza. Investigation of Preliminary Phytochemicals, Analgesic, Anti-Arthritic, Thrombolytic and Cytotoxic Activities of Begonia Roxburghii (Miq.) DC. Leaves. Med One. 2018 Feb 25; 3: e180001. https://doi.org/10.20900/mo.20180001

Copyright © 2020 Hapres Co., Ltd. Privacy Policy | Terms and Conditions