Location: Home >> Detail
TOTAL VIEWS
Adv Geriatr Med Res. 2019;1:e190008. https://doi.org/10.20900/agmr20190008
1 Ohio Musculoskeletal and Neurological Institute (OMNI), Ohio University, Athens, OH 45701, USA
2 Department of Biomedical Sciences, Ohio University, Athens, OH 45701, USA
3 Division of Geriatric Medicine, Ohio University, Athens, OH 45701, USA
4 Center for Cognitive Aging and Memory, Department of Clinical and Health Psychology, University of Florida, Gainesville, FL 32610, USA
5 Heritage Fellow, Translational Biomedical Science Program, Ohio University, Athens, OH 45701, USA
6 Laboratory for Computational Motor Control, Department of Biomedical Engineering, Johns Hopkins School of Medicine, Baltimore, MA 21218, USA
7 Division of Athletic Training, School of Applied Health Sciences and Wellness, College of Health Sciences and Professions, Ohio University, Athens, OH 45701, USA
* Correspondence: Brian C. Clark.
The capacity to move is essential for independence and declines with age. Limitations in mobility impact ~35% of adults over 70 and the majority of adults over 85. These limitations are highly associated with disability, dependency, and survival. More than 25-years ago the term “sarcopenia” was coined to highlight the age-related loss of muscle mass and strength with the assumption being that sarcopenia led to limitations in mobility. However, contrary to expectations, recent findings clearly indicate these variables only modestly explain limitations in mobility. One likely reason the current sarcopenia variables of muscle mass and strength do not discriminate, or predict, mobility limitations well is because they are heavily influenced by musculoskeletal mechanisms and do not incorporate measures reflective of the central neural control of mobility. Unfortunately, the precise central neural changes associated with aging that lead to decreased mobility are poorly understood. This knowledge gap has hampered the development of effective interventions for mobility limitations and the subsequent reduction of major functional disability for older adults. Here, we discuss the potential role of the motor control circuit of the dorsal basal ganglia as well as dopaminergic function in age-related reductions in mobility.
The capacity to move is essential for independence. Yet, this capacity declines with age. Limitations in mobility are classically characterized by slowness when walking and performing physical tasks [1,2]. Mobility limitations affect ~35% of adults over 70 and the majority of adults over 85 [2–4]. Limitations in mobility are highly associated with fall risk, disability, increased dependency, hospitalization, and mortality [1,5–9]. Slow gait speed, in particular, is strongly associated with survival in both men and women (Figure 1) [1]. Accordingly, age-related limitations in mobility are a significant public health burden with annual health care costs of $42 billion in the U.S. [10]. Our understanding of the mechanisms underlying, mitigation of and interventions to address mobility limitations remain very limited.
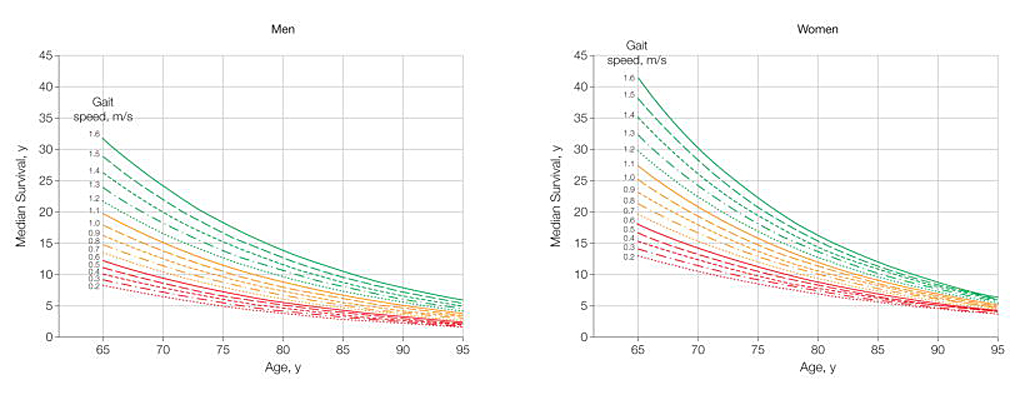 Figure 1. Predicted Median Life Expectancy by Age and Gait Speed. Reproduced with permission from Studenski et al., J Am Med Assoc, 2011 [1].
Figure 1. Predicted Median Life Expectancy by Age and Gait Speed. Reproduced with permission from Studenski et al., J Am Med Assoc, 2011 [1].
Prior research on mobility limitations in older adults has focused largely on factors related to musculoskeletal mechanisms and processes. The historical focus on the role of the musculoskeletal system in mobility likely arose from the heightened interest in sarcopenia, age-related loss of muscle mass [11]. It was posited that sarcopenia resulted in reduced muscle strength, and that the loss of muscle mass and strength was the major contributor to mobility limitations in older adults [11,12]. This premise led to a number of pharmaceutical companies pursuing compounds that mechanistically act on muscle (e.g., hypertrophy-inducing myostatin-inhibitors) with the goal being to enhance muscle and physical function [13]. The vast majority of these have failed to enhance mobility and physical function, due, in part, to the multifactorial determinants of mobility and physical function that extend well beyond muscle mass. In fact, recent findings indicate these “sarcopenia variables” of muscle mass and strength only modestly explain limitations in mobility [14]. For instance, the recent Sarcopenia Definitions and Outcomes Consortium group, who sought to develop diagnostic cut-points for low muscle mass and strength for identifying older adults at risk of mobility limitation (operationally defined as usual gait speed <0.8 m/s), concluded that grip strength expressed relative to BMI was associated with mobility limitation, but that low levels of appendicular lean mass was not [14]. Moreover, their identified cut-points had relatively low sensitivity (51% for men and 78% for women) and specificity (64% for men and 39% for women) for discriminating older adults with mobility limitation [14].
One reason that current sarcopenia variables of muscle mass and strength do not adequately discriminate or predict mobility limitations is because they are heavily influenced by musculoskeletal mechanisms. That is, these biomechanical based measures do not incorporate neural changes that occur with aging, which in turn also affect mobility.
In Figure 2, we highlight the strong relationship between a test of lower extremity motor function (the four square step test, which challenges motor planning and initiation as well as motor sequencing and recall [15,16]) and mobility, which was assessed based on a composite score from locomtor and non-locomotor tasks similar to that previously reported [17], in older adults (n = 82; 74.9 ± 6.7 years; 66% women). Indeed, work conducted over the past 10–15 years has provided strong evidence for the role of the nervous system contributing to mobility in aging (see [17–20] for recent review on this topic). Growing evidence suggests that aging is associated with slower and less ‘automated’ walking, with greater engagement of attention and cognitive resources [18,20]. Unfortunately, the precise neural mechanisms associated with aging that lead to decreased mobility are poorly understood. This knowledge gap has hampered the development of effective interventions for mobility limitations and the subsequent reduction of a major functional disability for older adults.
In 2012, a conference series was launched to facilitate the translation of research results into interventions that improve mobility for older adults [20]. The report from this conference series highlighted the evidential support for the central nervous system as an important contributor to mobility limitations in older adults without overt neurologic disease [20]. One of the key recommendations for future work was an increase in cross-disciplinary research to generate new ideas and address current methodological issues and barriers [20]. To this end, in this article we seek to bridge the gaps between the fields of geriatrics/aging systems, motor function, and neuroeconomics/decision neuroscience.
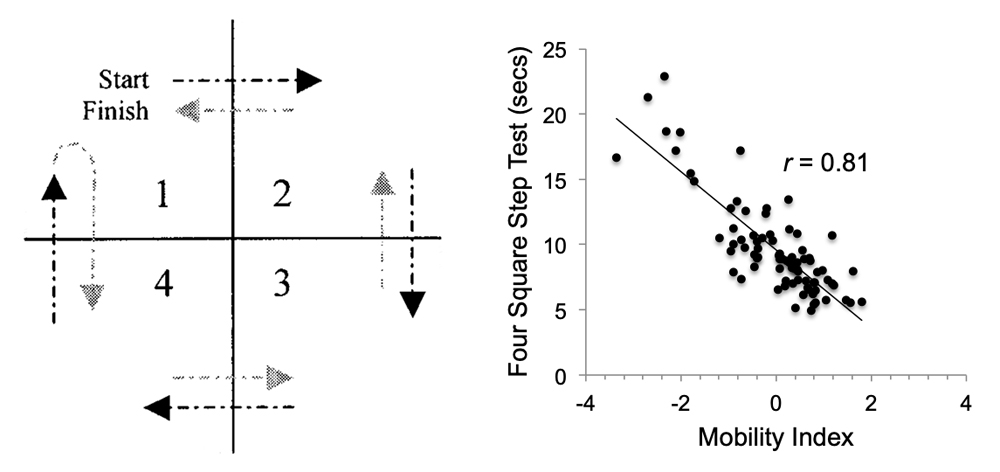 Figure 2. (A) The Four Square Step Test (4SST) is primarily a test of motor function as it heavily challenges motor planning and initiation as well as motor sequencing and recall. The subjects started in square 1, facing forward. The subject steps laterally into square 2, backwards to square 3, laterally to square 4, forward to square 1, backwards to square 4, laterally to square 3, forward to square 2, and laterally to square 1. (B) The 4SST is highly associated with mobility in older adults. Unpublished data from BC Clark [21].
Figure 2. (A) The Four Square Step Test (4SST) is primarily a test of motor function as it heavily challenges motor planning and initiation as well as motor sequencing and recall. The subjects started in square 1, facing forward. The subject steps laterally into square 2, backwards to square 3, laterally to square 4, forward to square 1, backwards to square 4, laterally to square 3, forward to square 2, and laterally to square 1. (B) The 4SST is highly associated with mobility in older adults. Unpublished data from BC Clark [21].
It is undeniable that the neural control of gait in older adults is multifactorial as gait is a highly complex skill [22,23]. Safe and effective negotiation of complex environments (i.e., community ambulation) requires the integration of external sensory information with neural networks, which involve cortical, subcortical, brainstem, and spinal cord structures [24]. It was long assumed that locomotion required minimal involvement of higher-order cognitive processes. However, in the last two decades we have learned that gait is not simply a motoric activity but critically dependent on cognition, and executive functions in particular, involving multiple brain resources [22]. Considering the multifactorial nature of mobility, clearly numerous neural substrates and circuits are implicated in age-related mobility declines [25]. In this article we discuss the potential role of the dorsal basal ganglia and its dopaminergic neuron function in age-related mobility limitations. This paper will present evidence to support the role of the basal ganglia in age-related mobility limitations. Its role is underappreciated within the context of mobility limitations in older adults, which is somewhat surprising when considering its involvement in the pathophysiology of movement disorders (e.g., Parkinson’s Disease) [26]. While the direct evidence for dysfunction of the dorsal basal ganglia being critically linked to mobility decline in older adults is limited, there is a strong theoretical framework to support its involvement that we present herein.
Animal studies have suggested that the dorsal basal ganglia circuit is critical for motivated, instrumental behavior [27]. This circuit consists of the striatum (caudate, putamen, and ventral striatum) as its input, and the internal globus pallidus and substantia nigra pars reticulate (SNr) as its output [28]. The striatum receives input from the vast majority of the neocortex (namely layer 5 projections via collaterals of descending cortiofugal fibers as well as intratelencephalic projections) and diverse thalamic nuclei [28]. The outputs of the basal ganglia shape activity in each of the multiple spinal-targeting pathways that regulate voluntary movement [29,30] via projections to the motor thalamus [31], pontine nuclei [32], and the superior colliculus [33].
Figure 3 illustrates anatomical organization of the basal ganglia [28]. The primary cortical inputs to the striatum are glutamatergic, producing excitation of striatal neurons. The principal neurons of the striatum are GABAergic medium spiny neurons which (in rodents) constitute more than 90% of all striatal neurons. These neurons have axons that project locally, as well as collaterals that project to other basal ganglia structures. At rest, the membrane potential of the medium spiny neurons is far from threshold (around −90 mV). This resting level is called the “down-state”. In response to converging excitatory input, for example from the cortex, the medium spiny neurons transition to an “up-state”, which can last hundreds of milliseconds, leading to production of spikes. A major modulator of the state of the striatum is dopamine. Arrival of dopamine affects the glutamatergic synapses, making it easier or harder to transition their up-state [34]. Thus, arrival of dopamine affects how the striatal neurons respond to the excitatory inputs that they receive from the cerebral cortex.
Progressive degeneration of midbrain dopaminergic neurons has been associated with deficits in the initiation, speed and fluidity of voluntary movement [35–37]. A slowing in movement initiation time and movement speed, as well as a reduction in movement amplitude, are some of the most profound effects of either acute or chronic disruption of dopamine signaling [38]. Specifically, a series of recent elegant studies have indicated that tonic activity (Figure 4) [38,39], as well as activity before action initiation (i.e., phasic changes in dopamine neuron activity) [38,39], in the dorsal striatum is critical for the regulation of movement providing strong evidence that dopaminergic input to the dorsal striatum is indispensable for the emergence of striatal activity that mediates adaptive changes in “movement vigor” (see Figure 4 legend for further discussion).
Control of movement vigor, i.e., reaction time and velocity, is strongly affected by activity in the basal ganglia [40]. In one of the basal ganglia output nuclei (SNr), cells reduce their activity when a more vigorous movement is to be performed, thus reducing the inhibition that they impose on downstream motor structures [41,42]. This modulation of activity in SNr is due to reward dependent activity of cells in the striatum [43], which in turn depend on the function of the dopaminergic system [44]. All species that have thus far been examined, including humans, move with greater vigor in response to promise of greater reward [45–51], and reduced vigor in response to promise of greater effort [51–53]. An event that generates a reward prediction error (experimental method to alter expected reward) strongly modulates production of dopamine [54,55]. A movement that takes place following such an event exhibits vigor changes: following a positive reward prediction error, movements are more vigorous, and following a negative reward prediction error, movements are slowed [56]. This suggests that in humans, as in rodents [39], a transient change in dopamine production results in a change in the vigor of the ensuing movement. However, occasionally a specific subject does not show this reward-dependent modulation of vigor in response to certain stimuli. That subject, at least in one case, was shown to also lack dopaminergic response to that stimulus, as well as lack of response in the caudate [44]. This suggests that lack of vigor response to reward may indicate a dysfunction in the dopaminergic system, as well as a dysfunction in the reward dependent activity of cells in the striatum.
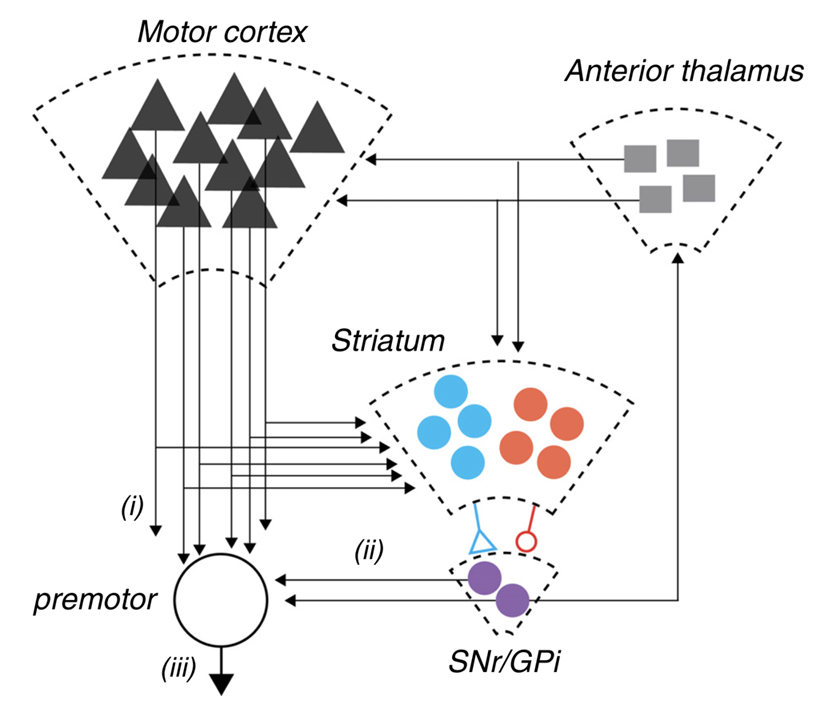 Figure 3. A schematic representation of the cortico-basal ganglia-thalamic circuit in mammals. Pathway i: corticofugal projection neurons from motor areas of cortex projecting both to brainstem and spinal cord targets. These same neurons elaborate extensive collaterals in the dorsal striatum. The dorsal basal ganglia is composed of a primary input structure, striatum, which contains two opponent populations of projection neurons (schematized in blue and red) that together provide an opponent projection onto the major output nuclei, substantia nigra pars reticulate (SNr) and internal globus pallidus (GPi) (purple). In addition, the dramatic reduction in projection neuron number from cortex to striatum to SNr is indicated by the decreasing size of the schematic representations. Finally, SNr output both projects feed-forward onto premotor neurons in the pontine nuclei and superior colliculus schematized by pathway ii as well as projecting recurrently to anterior thalamic nuclei (i.e., ventrolateral, ventromedial) that project back to cortex and striatum. Dudman and Krakauer have postulated that overt movement kinematics (pathway iii) result from the combination of motor command signals (pathway i) and reward processing control signals (pathway ii). Reproduced with permission from Dudman & Krakauer, Curr Opin Neurobiol, 2016 [28].
Figure 3. A schematic representation of the cortico-basal ganglia-thalamic circuit in mammals. Pathway i: corticofugal projection neurons from motor areas of cortex projecting both to brainstem and spinal cord targets. These same neurons elaborate extensive collaterals in the dorsal striatum. The dorsal basal ganglia is composed of a primary input structure, striatum, which contains two opponent populations of projection neurons (schematized in blue and red) that together provide an opponent projection onto the major output nuclei, substantia nigra pars reticulate (SNr) and internal globus pallidus (GPi) (purple). In addition, the dramatic reduction in projection neuron number from cortex to striatum to SNr is indicated by the decreasing size of the schematic representations. Finally, SNr output both projects feed-forward onto premotor neurons in the pontine nuclei and superior colliculus schematized by pathway ii as well as projecting recurrently to anterior thalamic nuclei (i.e., ventrolateral, ventromedial) that project back to cortex and striatum. Dudman and Krakauer have postulated that overt movement kinematics (pathway iii) result from the combination of motor command signals (pathway i) and reward processing control signals (pathway ii). Reproduced with permission from Dudman & Krakauer, Curr Opin Neurobiol, 2016 [28].
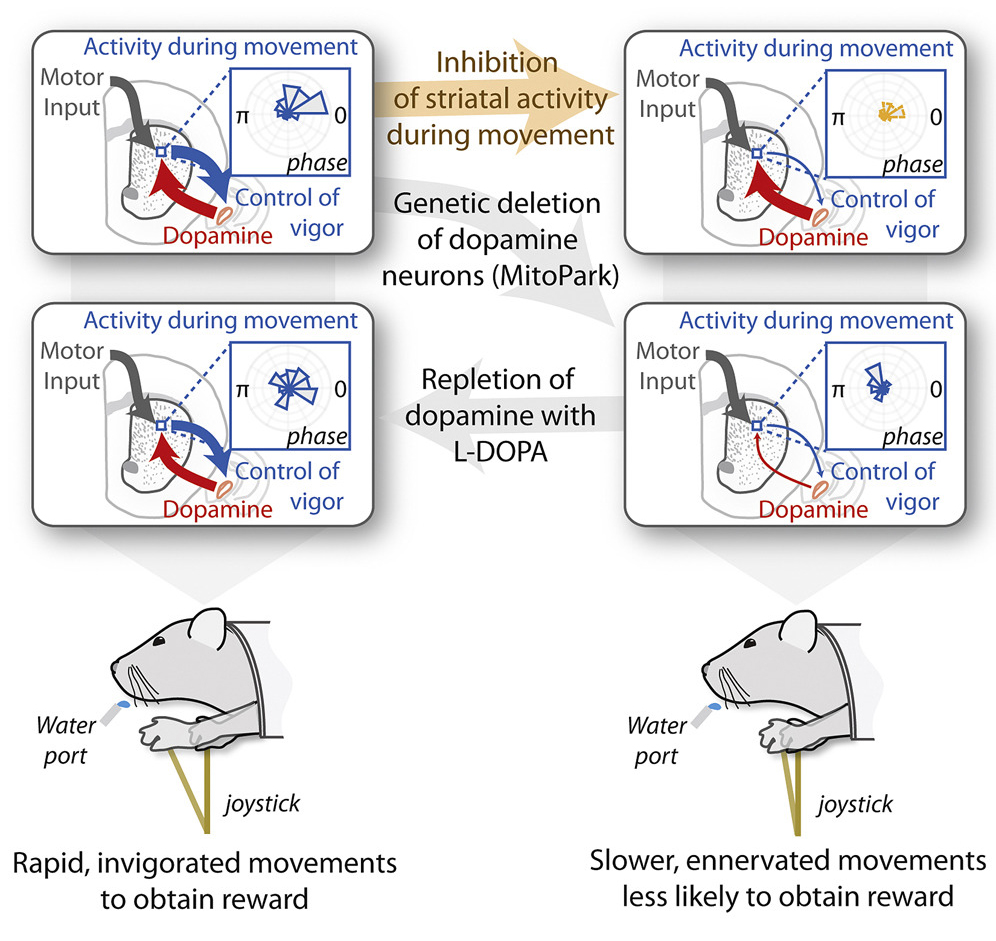 Dopamine is required for the neural representation and control of “movement vigor”. Movement vigor, a term that has largely arisen from the field of neuroeconomics, does not have a universally accepted definition per se, but is commonly used in the context of describing elementary, stimulus-driven movements, such as saccades and reaching. Within this context the operational definition is typically the inverse of the time from stimulus onset to movement completion, conditioned on distance [40,56]. This definition is based on the empirical observation that both reaction time and movement duration are influenced by the subjective value of the reward at the destination [45,46,48–50,57,58]. Panigrahi et al. reported that: (1) a mouse model of Parkinson’s disease produces a persistent reduction in effort; (2) the neural representation of movement vigor in striatum requires dopamine; (3) and acute suppression of striatal activity during execution enervates movement; and (4) dopamine repletion is sufficient to restore striatal activity and invigorate movement. Reproduced with permission from Panigrahi et al., Cell, 2015 [38].
Dopamine is required for the neural representation and control of “movement vigor”. Movement vigor, a term that has largely arisen from the field of neuroeconomics, does not have a universally accepted definition per se, but is commonly used in the context of describing elementary, stimulus-driven movements, such as saccades and reaching. Within this context the operational definition is typically the inverse of the time from stimulus onset to movement completion, conditioned on distance [40,56]. This definition is based on the empirical observation that both reaction time and movement duration are influenced by the subjective value of the reward at the destination [45,46,48–50,57,58]. Panigrahi et al. reported that: (1) a mouse model of Parkinson’s disease produces a persistent reduction in effort; (2) the neural representation of movement vigor in striatum requires dopamine; (3) and acute suppression of striatal activity during execution enervates movement; and (4) dopamine repletion is sufficient to restore striatal activity and invigorate movement. Reproduced with permission from Panigrahi et al., Cell, 2015 [38].
Studies of the aging human brain have shown that dopamine regulation is significantly reduced in old age via structural degradation including neuronal loss, fewer neuroreceptor sites, and a lack of transporter molecules (for review see [59]). For instance, more than 40 years ago the age-dependent decline of brain dopamine levels were noted in the basal ganglia, specifically the dorsal striatum (caudate nuclei and putamen) post-mortem [60]. In vivo imaging studies have confirmed these original findings [59]. More precisely, the availability of dopamine D1-like receptors declines in the human striatum at a rate of 7% per decade [61,62], with the D2-like family demonstrating a similar decrease in receptor density (~ 5–10%/decade) [63,64] and receptor binding potential (~6–8%) [63,65,66]. Despite a large number of studies documenting decreased striatal dopamine activity with aging, very little is known about its functional significance. There is a considerable amount of variation in the results of imaging studies that have measured the rate of striatal dopaminergic degeneration, which while due in part to differences in the binding characteristics of the tracers used [64], is arguably heavily driven by the heterogeneity of aging.
There are only a few studies that have examined the striatal dopaminergic contribution to age-related changes in gait, balance and other parameters of motor function [67–74]. Volkow et al. (1998) were the first to demonstrate that age-related decreases in brain dopamine activity in non-Parkinsonian older adults are associated with decline in motor function [69]. Here, 29 adults between 24–86 years underwent positron emission tomography and [11C]raclopride to assess dopamine D2 receptors. They observed that D2 receptor availability were significantly associated with a finger tapping task (number of index finger taps in 10-s) in both the caudate (r = 0.56, p < 0.01) and in the putamen (r = 0.56, p < 0.01) (Figure 5). In 2008, a similar cross-sectional study that involved subjects between 21 and 85 years old (n = 40) undergoing 2-β-carbomethoxy-3β—(4-fluorophenyl tropane (11C-β=CFT) dopamine transporter PET imaging, reported that after accounting for age and binding potential, that lower striatal dopamine transporter activity explained ~23% and 35% of the between subject variance in “comfortable pace” gait speed and cadence, respectively [67]. It should be noted that in this study the associations were largely driven by data from young adults (e.g., there were only 13 adults older than 70 years in the study and only three of these subjects had a gait speed <1.0 m/s (and none had a gait speed <0.8 m/s).
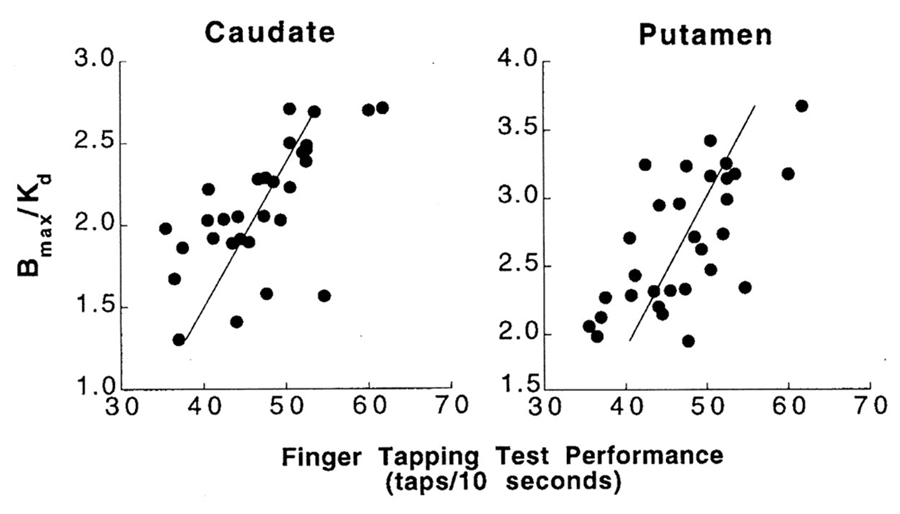 Figure 5. Association between Dopamine D2 receptor availability (Bmax/Kd) in the caudate and the putamen and performance on the finger tapping test. Reproduced with permission from Volkow et al., Am J Psychiatry, 1998 [69].
Figure 5. Association between Dopamine D2 receptor availability (Bmax/Kd) in the caudate and the putamen and performance on the finger tapping test. Reproduced with permission from Volkow et al., Am J Psychiatry, 1998 [69].
In more recent work taking advantage of advances in functional and structural brain imaging, more foundational sensorimotor capabilities that underpin mobility have been examined relative to basal ganglia function and structure. In 2012, Goble et al. used fMRI to examine age-related differences in the central processing of proprioceptive information by the nigrostriatal system, and examined its association with a behavioral measure of proprioceptive ability [68]. Here, 20 young (mean age: 26.1 years) and 20 older (mean age: 68.9 years) adults had their foot muscle spindles stimulated via tendon vibration while concomitantly measuring neural activation with functional magnetic resonance imaging. They noted a decrease in neural activity in a cluster of right putamen voxels for the older age group when compared with the younger age group. In follow-up experiments using diffusion tensor imaging, older (but not younger) adults with higher mean fractional anisotropy were found to have increased right putamen neural activity in response to spindle stimulation (r = 0.51 and 0.06, respectively) [68]. Age-dependent right putamen activity seen during tendon vibration was correlated with a behavioral test of proprioceptive ability measuring ankle joint position sense in both young (r = −0.39) and older (r = −0.40) age groups, and the relationship between the older adults joint position sense and neural activity in right putamen was mediated by structural differences (fractional anisotropy) within the right putamen [68]. The collective aforementioned findings provide proof-of-concept support for the potential role of dysfunction of the dorsal basal ganglia being critically linked to mobility limitations in older adults; however, care should be taken when interpreting these findings as they fail to address whether the neurophysiological changes map to the phenotype of mobility limited older adults.
Most recently, teams led by Rosso and Rosano have utilized epidemiologic approaches to investigate the relationship between dopaminergic genotypes and motor function in older adults [70,71]. In the first of these studies, Metti et al. (2017) examined the association between catechol-O-methyltransferase (COMT) genotype, a dopamine-regulating enzyme in the brain, particularly in the prefrontal cortex, and the change in time to walk 6-meters at one’s usual pace over a 10-year follow-up period. They observed that older adults with genotypes associated with lower tonic dopamine levels (i.e., the Val/Val and Met/Met genotypes) exhibited 9.5% and 13.9% slowing in gait speed, respectively, over a 10 year period when compared to those with the Met/Val genotype [70], which has been linked to increased levels of tonic dopamine [75]. In the second of these studies, Rosso et al. (2018) examined cross-sectional associations between mild Parkinsonian signs in older adults without overt neurological disease and COMT genotype as well as MRI-derived white matter hyperintensities (an indicator of cerebral small vessel disease) [71]. They observed that while COMT was not directly associated with the mild Parkinsonian signs, it did modify the effect observed between white matter hyperintensities and mild Parkinsonian signs, which they interpreted to suggest that the dopaminergic system might provide compensation for the negative effects of poor vascular health on motor function. Again, these findings provide proof-of-concept support for the potential role of dysfunction of the dorsal basal ganglia being critically linked to mobility limitations in older adults, but as with the other aforementioned findings are largely descriptive.
In addition to imaging modalities, striatal dopaminergic neuron function can be studied using in vivo electrophysiological techniques in humans. Specifically, a series of studies over the past 15-years have indicated that cortical plasticity to repetitive transcranial magnetic stimulation, in particular theta-burst stimulation (TBS), is largely dependent on striatal dopaminergic neuron function [76–82]. TBS uses bursts of high frequency brain stimulation (3 pulses at 50 Hz) repeated at intervals of 200 ms, and the classic paradigm employed is to examine the changes in motor evoked potentials before and after TBS with findings indicating that the degree of TBS-induced increase in the motor evoked potential is associated with striatal dopaminergic neuron cell loss [76–82]. To our knowledge, only one study has used this innovative approach in the context of aging [82]. Here, Di Lazzaro and colleagues examined the change in motor evoked potentials following TBS in eighteen healthy people whose mean age was 51.2 ± 17.9 years [82]. They divided these subjects into young adults (i.e., 20–40 years; n = 6), middle-aged adults (41–60 years; n = 6), and older adults (61–80 years). While they did not observe a statistically significant effect of age, likely due to the study being statistically underpowered, they did observe a robust difference between the older adults and their younger counterparts (Figure 6) providing further support to the notion of age-related impairments in striatal dopaminergic neuron function.
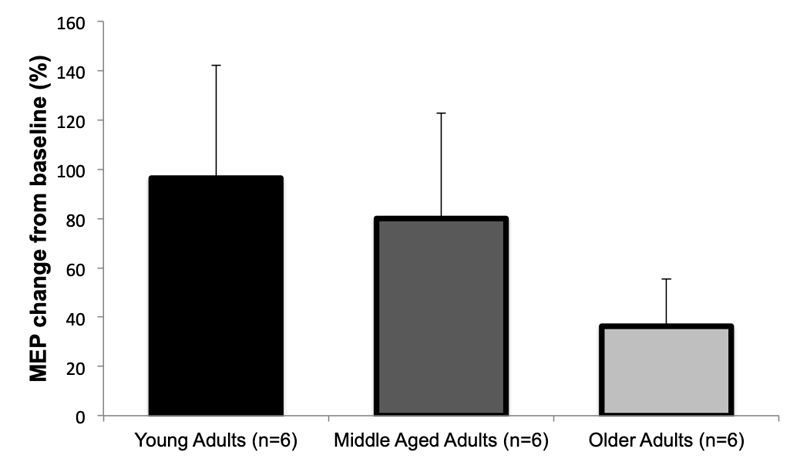 Figure 6. Mean increase in motor evoked potential (MEP) amplitude after intermittent theta-burst stimulation of healthy adults of different ages. Studies have indicated that cortical plasticity to theta-burst stimulation is largely dependent on striatal dopaminergic neuron function, and while these findings are not statistically different likely due to the small sample size, it should be noted that a robust mean difference between the older adults and their younger counterparts was observed providing further support to the notion of age-related impairments in striatal dopaminergic neuron function. Figure created from data presented in Di Lazzaro et. al, J Physiol, 2008 [82].
Figure 6. Mean increase in motor evoked potential (MEP) amplitude after intermittent theta-burst stimulation of healthy adults of different ages. Studies have indicated that cortical plasticity to theta-burst stimulation is largely dependent on striatal dopaminergic neuron function, and while these findings are not statistically different likely due to the small sample size, it should be noted that a robust mean difference between the older adults and their younger counterparts was observed providing further support to the notion of age-related impairments in striatal dopaminergic neuron function. Figure created from data presented in Di Lazzaro et. al, J Physiol, 2008 [82].
Movement vigor, a term that has largely arisen from the field of neuroeconomics, is commonly used in the context of describing elementary, stimulus-driven movements, such as saccades and reaching. Within this context the operational definition is typically the inverse of the time from stimulus onset to movement completion, conditioned on distance [40,56]. This definition is based on the empirical observation that both reaction time and movement duration are influenced by the subjective value of the reward at the destination [45,46,48–50,57,58]. In recent years, numerous studies have begun to conceptualize movement vigor as a trait-like attribute of individuality (see [40] for review). Among healthy individuals there is well-recognized diversity in movement vigor with some people tending to consistently move rapidly, whereas others tend to move slowly, as evidenced by their saccades (i.e., rapid, ballistic movements of the eyes that abruptly change the point of fixation) [83–85] and their reaching movements [83]. While classic theories in motor control suggest that differences in movement vigor reflect a speed-accuracy trade-off [86], recent data examining these competing hypotheses provided strong evidence indicating that movement vigor has no impact on end-point accuracy [87]. For instance, Reppert and colleagues (2018) reported that there is a strong positive relationship between vigor of arm and head movements in young adults (Figure 7), with movement vigor not altering end-point accuracy. That is, individuals who move their arm rapidly tend to also move their head rapidly with no negative impact on movement accuracy [83]. Findings of this nature have led to the assertion that movement vigor may be a trait, cutting across modalities of motor control, with the vigor with which a movement is performed being dependent on the subjective evaluation of reward, effort, and time [40,83]. As an example, when young adults were asked to press a key a number of times for a given amount of money, some preferred the low-reward/low-effort option, while others chose the high-reward/high-effort option [88]. A common aspect of individuality is our subjective preference in evaluation of reward and effort [88], and this prior work suggests that the degree to which people are willing to exert effort varies among healthy individuals. Interestingly, these differences are associated with between-subject differences in the neural circuits that evaluate reward and effort, particularly circuits that regulate dopamine transmission [87]. Changes in these circuits have been shown to alter patterns of decision making (e.g., depression: less willing to exert effort; amphetamines: more willing to exert effort), and they have also been shown to affect patterns of elementary movements (e.g., saccades are slower in the case of depression and faster in the case of amphetamine [87]). These findings suggest that the neural circuits that evaluate reward/effort and influence the decision of action selection partly overlap with the neural circuits that influence the decision of how fast to move. Findings of this nature have obvious implications to the conceptualization of the age-related limitations in mobility and physical function.
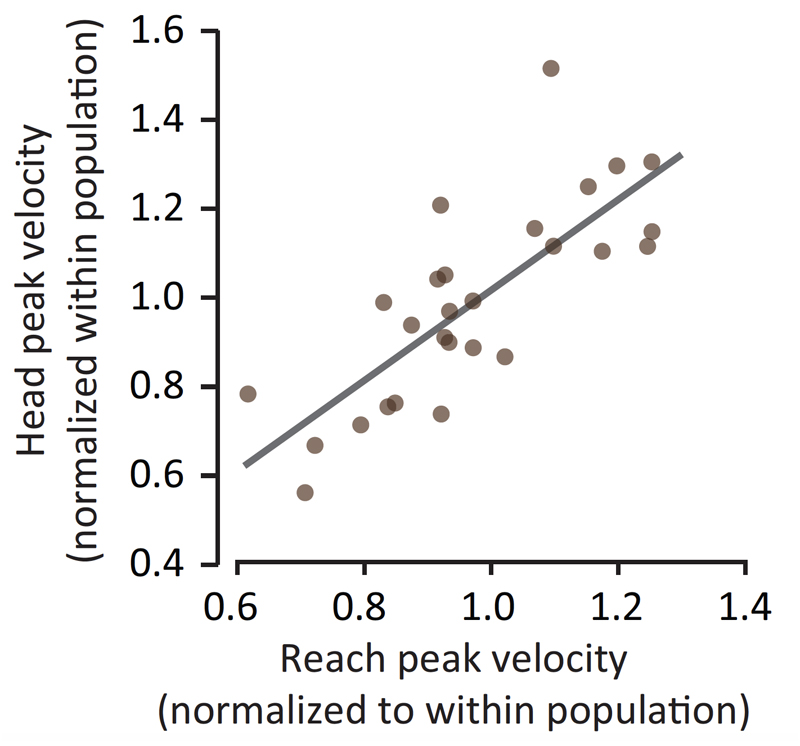 Figure 7. Young individuals with faster reaching movements also generate faster head movements. Findings of this nature have led to the assertion that movement vigor may be a trait, cutting across modalities of motor control, with the vigor with which a movement is performed being dependent on the subjective evaluation of reward, effort, and time [40,83]. Reproduced with permission from Shadmehr et al., Trends in Neurosci, 2019 [40]. (note: original data represented in this figure is from Reppert et al., J Neurophysiol, 2018 [83]).
Figure 7. Young individuals with faster reaching movements also generate faster head movements. Findings of this nature have led to the assertion that movement vigor may be a trait, cutting across modalities of motor control, with the vigor with which a movement is performed being dependent on the subjective evaluation of reward, effort, and time [40,83]. Reproduced with permission from Shadmehr et al., Trends in Neurosci, 2019 [40]. (note: original data represented in this figure is from Reppert et al., J Neurophysiol, 2018 [83]).
Corticostriatal functional connectivity studies have demonstrated connections between the dorsal striatum and motor related cortical areas [89–91]. Interestingly, cortical circuits related to cognition and executive function have also been shown to be from other distinct cortico-striatal networks that are involved in action selection, planning, and decision-making [27,92]. For instance, it has long been suggested that there are numerous basal ganglia-thalamocortical circuits, one of which is a dorsolateral prefrontal cortex circuit [92]. Moreover, it has recently been suggested that the human basal ganglia evolved from a circuit that—in lower vertebrates and some mammals—is sufficient to directly command simple or stereotyped movements to one that indirectly controls the vigor of goal-directed movements [28]. Lastly, a variety of inflammatory stimuli have been found to preferentially target basal ganglia function and lead to impaired motivation and motor activity [93,94]. This finding may support the notion that dysfunction of the dorsal basal ganglia is linked to mobility limitations in older adults as low-grade chronic inflammation has been shown to be an independent risk factor of impaired mobility in older persons [95]. Our suggestion that the speed at which an older adult moves may be a trait-like attribute in older adults reflecting an unwillingness to expend effort should not be taken to be in disagreement with the view that aging is associated with slower and less “automated” walking that requires greater engagement of attention and cognitive resources [18,20]. Rather, it could be compatible in that decline in dopaminergic output from the basal ganglia may lead not only to less goal-directed movement vigor but also reduced ability to integrate automatic motor programs (e.g., simple gait), leading to more cognitive resources for movement [96]. However, as cognitive ability also declines with age—this may limit older adults’ ability to “compensate” using this cognitive strategy for movement.
Limitations in mobility affect ~35% of adults over 70 and the majority of adults over 85. Limitations in mobility are highly associated with disability, dependency, and survival. There is a growing recognition that age-related mobility limitations are, in large part, due to degradations in the central neural control of mobility. Herein, we discuss the potential role of the motor control circuit of the dorsal basal ganglia and its dopaminergic neuron function in age-related mobility limitations. While relatively little work has directly investigated the role of the motor control circuit of the basal ganglia and its dopaminergic neuron function in age-related mobility limitations, there is a strong theoretical foundation for its potential involvement. Borrowing on recent findings in the neuroeconomics literature there is mounting evidence suggesting that the willingness to exert effort varies between people and these differences are correlated with differences in the neural circuits that evaluate reward and effort, particularly dorsal striatal circuits that regulate dopamine transmission. Accordingly, we postulate that the speed at which an older adult moves (e.g., gait speed, which has been suggested to serve as a “sixth vital sign” for older adults [97,98]), may be a trait-like attribute in older adults reflecting an unwillingness to expend effort. Overall, the integration of the findings discussed herein suggest that future research should investigate the contribution of baseline and/or early “movement vigor” and the associated reward mechanisms to age-related limitations in mobility and physical function. Developing a better understanding of the neural basis of mobility limitations in older adults may help identify neurotherapeutic targets for enhancing mobility.
This work was supported in part by a grant from the NIH’s National Institute on Aging (R01 AG044424 to BC Clark), and National Institute on Neurological Disorders and Stroke (5-R01-NS078311 and 1-R01-NS096083 to RS).
In the past 5-years BCC has received research funding from the NIH, Regeneron Pharmaceuticals, Astellas Pharma Global Development, Inc., RTI Health Solutions, Biophytis, and the Osteopathic Heritage Foundations. In the past 5-years BCC has received consulting fees from Regeneron Pharmaceuticals, Abbott Laboratories, and the Gerson Lehrman Group. Additionally, BCC is co-founder with equity, and serves as the Chief of Aging Research, of AEIOU Scientific, LLC. The other authors report no conflicts of interest.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
37.
38.
39.
40.
41.
42.
43.
44.
45.
46.
47.
48.
49.
50.
51.
52.
53.
54.
55.
56.
57.
58.
59.
60.
61.
62.
63.
64.
65.
66.
67.
68.
69.
70.
71.
72.
73.
74.
75.
76.
77.
78.
79.
80.
81.
82.
83.
84.
85.
86.
87.
88.
89.
90.
91.
92.
93.
94.
95.
96.
97.
98.
Clark BC, Woods AJ, Clark LA, Criss CR, Shadmehr R, Grooms DR. The Aging Brain & the Dorsal Basal Ganglia: Implications for Age-Related Limitations of Mobility. Adv Geriatr Med Res. 2019;1:e190008. https://doi.org/10.20900/agmr20190008

Copyright © 2020 Hapres Co., Ltd. Privacy Policy | Terms and Conditions