Location: Home >> Detail
TOTAL VIEWS
Crop Breed Genet Genom. 2025;7(3):e250010. https://doi.org/10.20900/cbgg20250010
School of Forestry and Horticulture, Southern Illinois University, Carbondale, IL 62901, USA
Horseradish (Armoracia rusticana Gaertner, Meyer & Scherbius) is often times described as sterile because the flowers it produces does not generally form pods filled with seeds or only develops seed in very limited quantities. Some past research have concluded that horseradish is highly sterile due male sterility resulting from non-viable pollen, but another possible mechanism for the perceived improper seed set and development in horseradish is self-incompatibility (SI), related to mate limitation. Thus, a study was designed to further document horseradish pollination and fertilization by evaluating the self- and cross-compatibility of several horseradish clones collected from different locations in the world with two Illinois, USA tester clones (15K as pollen acceptor and 9705 as pollen donor). All clones evaluated had viable pollen, indicating that pollen sterility should not have been an issue in preventing pollination and eventual seed development in these clones. Most horseradish clones obtained from Europe and Russia provided self-rejection responses to their own pollen, although three accessions evaluated did have some minimal capsule set and seed development (874A from England; 784A from Moscow, Russia; and 866A from Laufener, Germany). Most clones evaluated also provided compatible pollen with 15K for capsule formation, ranging from 39% to 100%. Additionally, most clones were compatible with 9705 pollen, although some had higher compatibility success for capsule set and development. This research indicates that mate limitation appears to have played a significant role in this crop being originally identified as sterile from the lack of seed production. If the most widely grown clone in a particular locale or region is SI, then horseradish clones in that area would appear sterile since the only mate nearby would be itself. This research using horseradish clones from various regions of the world further suggests that the once perceived sterility in this plant species is most likely due to SI pollination and fertilization systems that are typical for the Brassicaceae family.
Horseradish (Armoracia rusticana Gaertner, Meyer & Scherbius, Brassicaceae) is a perennial herb in the Brassicaceae family, cultivated for its edible, pungent root [1,2]. Horseradish is a flavorful pungent herb that has been cultivated and used for centuries to enhance the flavor of food, aid in digestion, and improve human health [3]. It is commercially grown in many cold-temperate regions of the world, with the main areas of commercial production found in Europe and North America, although smaller production regions occur in Asia, South Africa, South America, and Russia [4,5].
Horseradish is highly heterozygous and will not reproduce true from seed in those genotypes that are self-compatible (SC) [4,6]. Thus, horseradish is vegetatively propagated using small root pieces to maintain uniformity of commercial plantings [1,2]. Moreover, the development of new improved cultivars has been somewhat limited by the lack of viable seed resulting from low fertility of horseradish flowers [1,6]. However, Walters [5,6] indicated that seed can easily be produced from many of the crosses made among those cultivars, breeding lines or other germplasm materials used in the Illinois, USA breeding program, although the number and viability of seed obtained differs among the specific crosses made due to sexual compatibility among the different clones.
Horseradish Pollination and FertilitySince horseradish clones are highly heterozygous and maintained through asexual propagation procedures [2,5,6], sexual reproduction in horseradish has evolved to have low fertility, presumably due to the costs associated with sex which is not needed to perpetuate this asexual species [7]. Although horseradish is known to profusely produce flowers in nature, this plant generally produces none or few viable seed [1,8,9,10,11]. Horseradish is often times described as sterile because it does not normally form capsules following flower pollination or only develops capsules with seed in very limited quantities [6]. For many years, horseradish flowers were believed to be sterile [12], since only a few viable seed were produced either under natural conditions by wind or insects, or by hand-crossings. Thus, due to this low fertility and sterility, the only way to improve horseradish was to select and plant root cuttings from the most desirable plants [13].
Horseradish and Mate LimitationMale sterility [8,10,11,14] and mate limitation [6] have both been proposed to explain why few capsules develop from horseradish flowers, and the none to low amounts of viable seed in those that do form. Stokes [8] first reported that only about 60% of horseradish pollen was functional, and more recently, Winiarczyk [10] concluded that horseradish is highly sterile due to male sterility resulting from non-viable pollen. Male sterility, which is the failure to produce viable male gametes, has long been suspected as the primary obstacle to formation of viable seeds in horseradish [8,10,11]. Another possible underlying mechanism for poor viable seed development in horseradish is Self-incompatibility (SI) [6,8], which is related to mate limitation. In the case of horseradish, mate limitation would arise in SI clones that cannot find another genetically dissimilar horseradish plant in close proximity to fertilize flowers. Self-incompatible clones and the suitable males required to induce successful fertilization and viable seed formation have difficulty locating each other. Since horseradish is propagated asexually by root cuttings, a single horseradish clone having superior root characteristics can often predominate a regional agricultural production system [6]; and if the most important clone in a particular locale or region is SI, then horseradish plants in that area would appear sterile since the only mate in the region would be itself [15]. Moreover, in Poland, Winiarcyzk [10] indicated that cross-breeding horseradish plants from similar geographical regions resulted in the production of only a few viable seeds, while greater seed numbers can be obtained when cross-breeding plants from different geographical areas. Additionally, there seems to be some differences in pollination and fertilization systems among different types of horseradish, as ‘Common’ types of horseradish are known for their frequent failure to develop viable seed, with flowers often aborting before opening [16]. Horseradish pollination and fertilization systems are still not fully understood, but SI is a possible mechanism for the poor or lack of capsule and seed development in horseradish.
Horseradish as a Neglected SpeciesThere is a lack of basic biological and genetic information for horseradish, due to the lack of research support expended to study this high value specialty crop [5]. Horseradish pollination and fertilization is one such area that has incomplete information available to fully understand the mechanisms involved compared to many other species in the Brassicaceae family that have been extensively studied [17]. Pollination in most Brassicaceae exhibits a Sporophytic SI system where S alleles expressed on the stigmatic surface interact with S alleles in the pollen coat facilitating pollen acceptance or rejection [18,19]. Inhibition of self-pollen to germinate on a stigmatic surface or production of non-functional pollen tubes occur when these alleles match, with stigmatic papilla blocking pollen grain hydration and pollen tube growth [20]. Walters et al. [6] indicated that a similar SI system may be involved in preventing self-pollinations in horseradish. Self-recognition responses were shown to vary between Illinois USA horseradish clones, as certain clones recognized and rejected their own pollen, while others accepted their own pollen and eventually produced copious amounts of seed. Thus, horseradish grown for hundreds of years or longer in geographically isolated areas may have played a significant role in this crop originally being identified as sterile. Therefore, a study was designed to provide a greater understanding of the pollination and resulting fertilization systems of horseradish by documenting the sexual reproduction among several clones collected from different locations in the world.
The Horticulture Research Center Greenhouse at Southern Illinois University—Carbondale was used during March to May 2019 and 2020 to identify clones collected at various locales in the world as either SC or SI based on their ability to form pods with viable seeds following hand-mediated self-pollinations and pollen viability analyses. Additionally, cross-compatibility of these clones were also evaluated with two Illinois, USA tester clones (15K as pollen acceptor and 9705 as pollen donor). The Horseradish Growers of Illinois maintains a diverse sample of clones collected from various locations in Europe and Russia, with most collected in the 1960s [13,21], although three accessions evaluated (AM 446, ‘Austrian’, and ‘Hungarian’) were more recently collected. Eleven clones were from Europe and Russia, while two commercial Illinois, USA clones (15K and 9705), and an older commercial clone, Big Top Western, were also included (Tables 1 and 2).
Flowers only form on horseradish plants that have new, current year flower primordia, which develop after a season of field growth. Thus, each clone used in the study was grown under commercial field conditions in Caseyville, Illinois, USA during both 2018 and 2019 to obtain crown roots that would produce flowers the following spring. These roots with flower primordia were then placed in cold storage (2–5 °C) for ~3 months to induce flower development. These crown roots were then removed from cold storage in early March with four (from a specific clone) placed into a 7.6 L plastic pot (22 cm dia.) containing a mixture of 1 part soilless Berger BM1 (peat moss, perlite, vermiculite) mix (Saint Modeste, Quebec, Canada) and 1 part sand/silt loam soil (steam-sterilized at 70 °C for 6 h). About 20 g of the insecticide Marathon 1% G (imidacloprid 1% granular; Olympic Horticultural Products Inc., Mainland, PA, USA) and 50 g of a slow-release fertilizer Osmocote® 14-14-14 (N-P-K; Scotts-Sierra Horticultural Products Co., Maryville, OH, USA) were placed on top of the soil in each pot once horseradish plants had emerged. All containers with horseradish plants were watered once or twice daily, depending upon the moisture needs of the plants. Greenhouse temperatures in the early spring averaged approximately 25 to 30 °C day and 12 to 18 °C night.
Experimental SetupThe self- and cross-compatibility experiments were setup with 14 treatments (horseradish clones) per greenhouse bench placed in a randomized complete block design with 4 replications. Four horseradish crowns of a particular clone were grown in each pot, with the flowering stem from one used in the selfing evaluation and two other flowering stems from other crowns utilized for the cross-compatibility tester clones assessment.
Hand-Pollinations and Data CollectionHorseradish plants began producing flowers approximately three to four weeks after planting. Once flowering had begun (late-March), self-pollinations were made on the 14 clones described earlier and cross-pollinations were made using horseradish clones obtained from Europe and Russia, and Big Top Western, as male donors for 15K and as female parents with 9705. However, flowers used as the female parent for cross-pollinations were emasculated on all clones that were not totally SC a day prior to flowering to prevent self-pollen contamination. All pollinations were made by hand, between 9 am and 12 pm each day during a 3- to 4-week period (late-March to late-April), with 100 pollinations made on a clonal flowering stem for each self- or cross-pollination combination. Both experiments were set up as completely randomized designs with 4 replications each year. The number of capsules that developed from pollinations in each experimental unit was enumerated from approximately mid-April to mid-May. Data were also collected for those fruit pods that were undersized and thin, as well as those that were enlarged and plump. Also, prior to fruit dehiscence, seeds were visually examined from each capsule to determine potential viability (e.g., seed size and plumpness).
Pollen Viability AnalysisOnce all horseradish clones were blooming, fresh flowers were collected from 9 to 11 am. The dehiscent anthers were rubbed against a glass slide to dislodge pollen grains with 1–2 drops of Calberla’s fluid placed on the slide to stain pollen [22]. A coverslip was placed on top of the slide and placed in darkness for 24 h to allow staining [23]. Slides were placed under a light microscope at 20× and 40×. Pollen grains were counted as viable if the exine turned pink, the grain was hydrated, and granular cytoplasm was observed underneath the exine. Five open flowers were randomly collected from each clone in each experimental unit. Pollen grains were placed on a slide with staining solution with 50 randomly assessed within a few days after staining. Pollen viability was only obtained by observing the staining reaction with percentage of viable pollen obtained by dividing number of stained pollen grains by the total number examined and then multiplying by 100. Comparisons to verify the staining procedure was made with non-viable horseradish pollen collected several days earlier that was allowed to dry.
Statistical AnalysisData were first subjected to analysis of variance procedures using the general linear models procedure of SAS (version 9.4; SAS Institute, Cary, NC, USA) appropriate for a completely randomized experimental design to compare the effectiveness of horseradish self-pollinations, and cross-pollinations with 15K and 9705. Tukey’s test was used to separate treatment differences at p ≤ 0.05.
All horseradish clones evaluated had 100% viable pollen according to the pollen viability analysis. Stained pollen of several horseradish clones evaluated are shown in Figure 1. All pollen grains from the clones evaluated were viable as the exine turned pink, the grain was hydrated, and granular cytoplasm was observed underneath the exine. The non-viable horseradish pollen used for comparison did not show a staining reaction indicating that it was not viable. This indicates that pollen sterility should not have been an issue in preventing pollination and eventual seed development in the horseradish clones evaluated, but that pollen recognition and pollen tube growth were probably most critical.
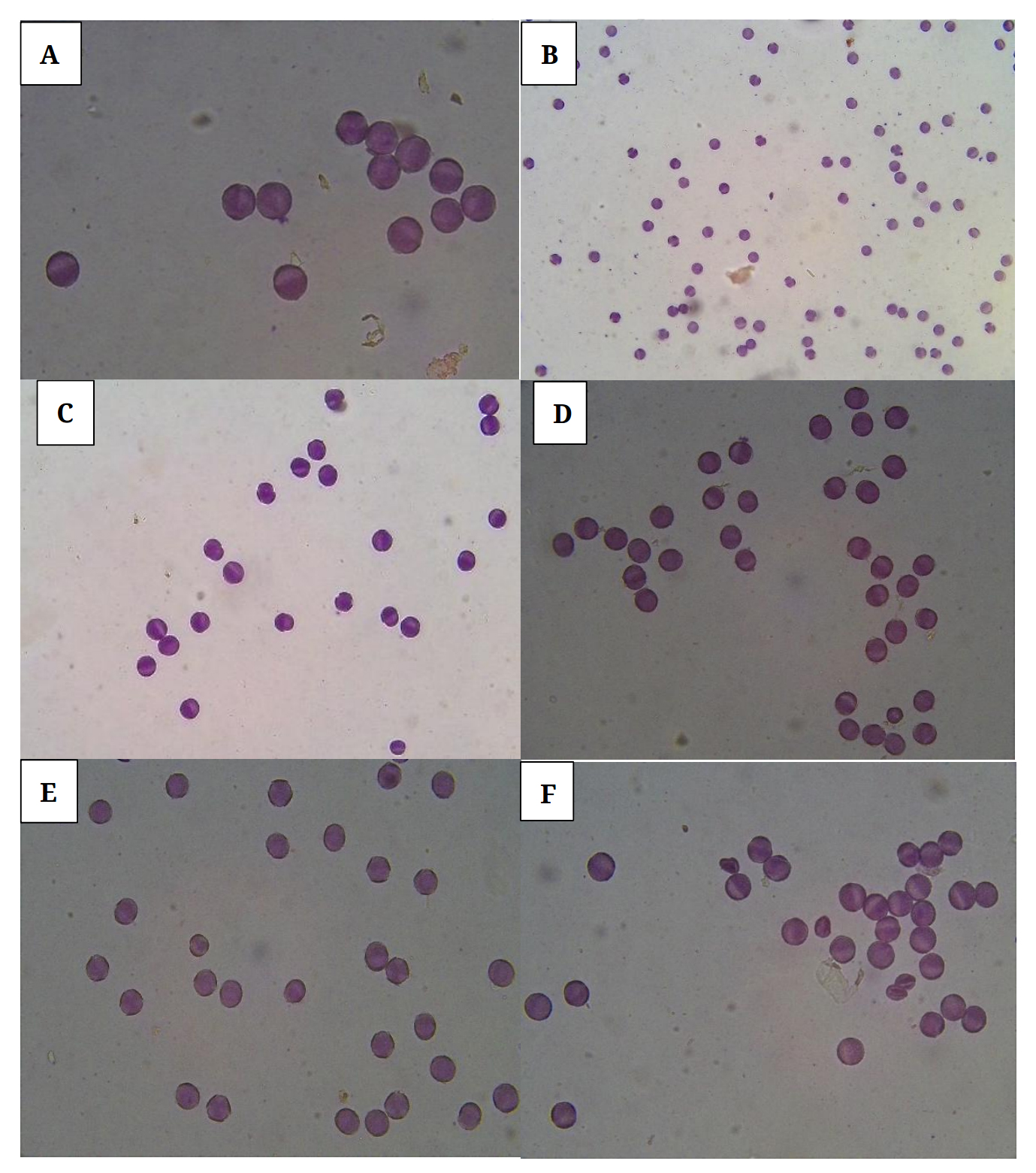 Figure 1. Horseradish pollen from a few selected clones stained with Calberla’s fluid at various magnifications: (A) 761A (Poland) at 40×, (B) 9705 (Illinois, USA commercial variety) at 20×, (C) AM 446 (Romania) at 40×, (D) 764A (England) at 40×, (E) 866A (Germany) at 40×, and (F) Austrian (Austria) at 40×. Notice all pollen grains show viabilty from the staining procedure utilized.
Figure 1. Horseradish pollen from a few selected clones stained with Calberla’s fluid at various magnifications: (A) 761A (Poland) at 40×, (B) 9705 (Illinois, USA commercial variety) at 20×, (C) AM 446 (Romania) at 40×, (D) 764A (England) at 40×, (E) 866A (Germany) at 40×, and (F) Austrian (Austria) at 40×. Notice all pollen grains show viabilty from the staining procedure utilized.
Horseradish clones from a wide geographical area in Europe and Russia were evaluated for the ability to accept or reject their own pollen by observing capsule set with viable seed, and included accessions from Czech Republic, England, Germany, Hungary, Poland, Romania, Russia, and Slovenia. The horseradish clones obtained from these different locations provided most self-rejection responses to their own pollen, which resulted in little to no capsule and seed formation with most were classified as completely SI (Table 1). These SI clones from Europe and Russia included a range of plant types based on leaf shape and orientation (Table 1), with five accessions that were classified as Common types, four as Bohemian types, and two as Big Top Western types.
Some accessions did produce capsules with seed, although only one provided a highly SC self-reaction to their own pollen (Table 2). The Illinois, USA commercial variety 9705 had high self-pollination success with 95% capsule set from its own pollen. Well-formed capsules with 6 or more seed in each was observed for 9705. This variety was developed for the Illinois, USA horseradish industry in the 2000s and resulted from outcrossing an accession 758A collected from Ribnica, Slovenia with an unknown male. Additionally, three accessions evaluated did have some minimal seed development. The highest self-pollen capsule set of the three, 874A, is a Bohemian type from England that provided capsules with limited amounts of seed in 41% of self pollinations. Two others, 784A, a Bohemian type from Moscow, Russia, and 866A, a common type from Laufener, Germany were termed highly SI, with only 18% and 15% success in producing capsules with seed from self-pollinations; and in both cases, the capsules were small and underdeveloped with less than 3 small seed produced on average in each. All other clones evaluated were completely SI. This includes 15K, a once widely grown commercial variety in Illinois, USA, which produced underdeveloped capsules with no seed formation. Additionally, Big Top Western (or accession 1430A) was also completely SI and produced long, small and thin empty capsules when self-pollinated. Two SI clones, 785A from Maikop, Russia and 756A from Sostro, Slovenia are depicted in Figure 2, showing no pod development from spent self-pollinated flowers.
Most clones evaluated provided pollen that was compatible with 15K for capsule formation, although some had more compatibility success for capsule set than others (Table 2). Those that provided pollen for >90% capsule set success with 15K were 874A, 784A, and Austrian, while 756A, AM 446, and 785A had 81%, 78% and 70% success, respectively. Several other clones (761A, 864A, 866A, 553A, and Hungarian), ranged from 39% to 65% for capsule set success, indicating that most clones evaluated provided pollen that had some recognition to allow capsule set and some seed development when placed onto 15K stigmas. The horseradish clone 864A was observed by the author to have low amounts of visible pollen compared to many other clones evaluated (Table 1) which may have contributed to its poor success of pollen compatibility with 15K. Additionally, most clones evaluated were compatible with 9705 pollen, although there were some variability for capsule set and development among clones. Those with >90% capsule set success rate with 9705 pollen were 874A, 866A, 553A, 756A, 761A, Austrian, and Hungarian. In comparison, Big Top Western, 784A, and AM446 had 85%, 77%, and 62% capsule set success with 9705 pollen, respectively, while only 24% and 29% success were obtained for 785A and 864A, respectively. The cross of Big Top Western with pollen from 9705 is depicted in Figure 3 and shows the development of large pods, which eventually resulted in an overall 85% capsule set. This indicated that 9705 provided compatible pollen that was not recognized on the stigmatic surface of most clones evaluated which generally allowed capsule formation and seed set.
Horseradish has been grown for centuries in Eastern Europe and Russia, and data presented here indicated that clones collected from this part of the world differed in their ability to either accept or reject their own viable pollen, with only a few forming capsules with viable seed from self-pollination. Additionally, all horseradish clones evaluated in this study, regardless of location, also produced highly viable pollen indicating that male sterility was not the issue causing reduced capsule and seed set. This supports previous work for pollen viability that shriveled, empty and collapsed exines were rare or non-existent in Illinois, USA horseradish clones [6]. Additionally, Sampliner and Miller [24] found that few viable seed were produced by horseradish plants found in its native ranges in Eastern Europe, which indicate that the surrounding plants in a given cultivated population are most likely genetically similar. Winiarczyk et al. [10] also reported from Poland that slightly greater numbers of horseradish seed can be obtained through cross-breeding plants from differing geographic areas, although the use of cross-breeding still only results in the production of few seed. Walters et al. [6] also found 74% of Illinois, USA horseradish clones evaluated had some level of SI and >80% of self-pollinations resulted in some type of abnormal capsule development containing no seed or a large portion of non-viable seed. Other work reported that SI reactions in horseradish stigmas were typical of SI responses in other Brassicaceae species [6,11]. If a pollen grain is self-incompatible, the stigmatic papilla rejects it by blocking pollen grain hydration and pollen tube growth [20]. Moreover, based upon pollen tube germination and growth studies in SI clones, Walters [6] also indicated that pollen either fails to penetrate the stigma, or if penetration occurs, pollen tubes fail to reach the ovary or ovule. Although horseradish was originally thought to be totally pollen sterile from the lack of seed production [1], this proposed SI system appears to be the most likely scenario.
Horseradish Reproductive System Evolution Asexually-Propagated SpeciesHorseradish is an asexually propagated crop perpetuated by the selection and replanting of root cuttings from plants having the highest quality roots and/or yields. This crop species may have gradually lost its ability to effectively reproduce in nature by sexual means through the course of evolution [10]. Asexual species enjoy various growth benefits because they seemingly rid themselves of the costs associated with sexual reproduction, or the so-called cost of sex [7]. The evolutionary loss of sexual reproduction in natural populations of A. rusticana is similar to many other plant species that reproduce both generatively and vegetatively, with a preference for asexual reproduction occurring over time [25,26,27]. The cost of sexual reproduction is essentially a problem of resource allocation. Since the retention of desirable characteristics of A. rusticana can be achieved through vegetative procedures, sexual selective pressures are not as important compared to other plant species that depend on sexual reproduction for adaptation because they are not required for perpetuation of this species [7,27].
Influence of Limited Sexual Reproduction in Horseradish GenotypesIn a highly clonal species like horseradish, low levels of sexual reproduction may be a by-product of increased vegetative and/or root growth [6]. An asexual female would thus have a significant reproductive advantage, and if both reproductive forms compete for the same resources, clonal females should rapidly displace all sexual females [7]. Often times, we have observed that under greenhouse conditions clonal offshoots frequently arise in leaf axils of certain horseradish clones that do not produce viable seed (Figure 4). Furthermore, Winiarczyk and Bednara [11] indicated that the continuous vegetative reproduction of horseradish favors the accumulation of mutations, limits the possibility of selection, and prevents the transfer of genes among different genotypes; and, the total elimination of sexual reproduction will most likely have a negative influence on ecological and genetic characters of horseradish over time. Thus, limited gene transfer resulting from reduced sexual reproduction will definitely decrease the potential selection of more desirable horseradish genotypes for an ecosystem over time.
 Figure 4. New plantlet development in leaf axils of clones that are completely self-incompatible: (A) 903 (a commercial Illinois, USA grown clone) and (B) 761A (an accession collected from Drazgow, Poland). Notice no viable capsule formation but the plantlets in leaf axils easily form roots when removed from the mother plant and placed in moist soil.
Figure 4. New plantlet development in leaf axils of clones that are completely self-incompatible: (A) 903 (a commercial Illinois, USA grown clone) and (B) 761A (an accession collected from Drazgow, Poland). Notice no viable capsule formation but the plantlets in leaf axils easily form roots when removed from the mother plant and placed in moist soil.
SI is a pollination and fertilization system that promotes cross-fertilization, which allows plants to maintain genetic diversity [28]. It appears that all evolution streams within the Brassicaceae family contain some self-incompatible species [29]. Many wild plants and domesticated crops of the Brassicaceae exhibit sporophytic SI to prevent self-pollination [17]. This system has evolved to allows stigmas to recognize and discriminate against “self” pollen, which prevents self-fertilization and inbreeding [30]. A balance between outbreeding and inbreeding provides an advantage for the survival and evolution of a species (such as A. rusticana), since inbreeding will improve its survival at any point in time and outbreeding enhances the survival of the species over time. We have noticed that while most clonal accessions evaluated in this study are completely SI, a few are classified as highly or partially SI and will produce some seed (Table 1); and, thus, those that are highly or partially SI would have the ability to produce some self-seed and allow a particular clone to be perpetuated via new offspring and survive in difficult times, while, other clones that were completely SI would most likely no longer exist with the passage of time. Bateman [29] indicated that the Brassicaceae has a high level of self-sterility, and those that are perennials in habit, like horseradish, tend to be self-sterile. Moreover, SI throughout the Brassicaceae is most likely due to the same mechanism [29]. Horseradish is a species in the Brassicaceae that appears to act in a similar manner to many other members in this family that recognize and reject ‘self’ pollen [6]. This study suggests that this sporophytic SI system acts similarly in horseradish as other Brassicaceae members, which resulted in this species originally being described as male sterile.
Human Selection of Horseradish and Mate LimitationMate limitation of horseradish appears to have played a significant role in this crop originally being identified as sterile from the observed consistent lack of seed production. Repeated propagation of a particular horseradish clone most likely resulted in the perceived sterility due to SI mechanisms preventing fertilization between genetically identical/similar clones [24]. The widespread use of a specific horseradish clone at a particular geographic locale would appear sterile, since it would most likely have been SI and would reject its own pollen. Although horseradish clones tend to differ genetically among different villages in Eastern Europe, those within a village tend to have similar genetic profiles (Miller, A.J., St. Louis University-USA, personal communication, 2025), indicating the importance of perceived sterility in horseradish and likelihood of mate limitation. The author’s perception is that mate limitation played a significant role in horseradish grown in geographically isolated areas being originally identified as sterile.
Horseradish is an asexually propagated crop that has been perpetuated by the selection and replanting of root cuttings from plants having the highest quality roots and yields. Compared to other types of horseradish, ‘Common types’ are known to produce high-quality and larger roots compared to other types [2]. These horseradish types were probably most preferred for cultivation and agricultural use by humans due to these characteristics, and based upon the data presented, all evaluated in this study were either highly or completely SI in all geographic areas of Europe in which they were collected. Additionally, ‘Common types’ most likely became more distributed all over Europe when this horseradish type was chosen for its outstanding root quality characters. All ‘Common types’ evaluated in this experiment were completely SI, except a clone from Laufener, Germany, which had a 15% success of forming a seed capsule from self-pollination indicating a high SI reaction. Thus, the perception of sterility in horseradish is likely due to sporophytic SI and mate limitation.
Horseradish is locally sterile, but globally compatible, meaning that horseradish clones from contiguous geographical locales have similar genetic backgrounds translating to SI, whereas those from diverse geographical locations are more genetically different, allowing for some cross-compatibility among clones. Thus, mate limitation of horseradish appears to have played a significant role in this crop originally identified as being sterile from the lack of seed production. This evaluation of self- and cross-pollination recognition responses comparing horseradish clones from various regions of the world along with other research by this author [6] indicates that the once perceived sterility in this plant species is most likely due to sporophytic SI pollination and fertilization systems that are typical for the Brassicaceae family.
All data generated from the study are available in the manuscript.
The author would like to thank the staff at the Southern Illinois University-Carbondale Horticultural Research Greenhouse and the graduate and undergraduate students who helped with this research over the years that it was conducted.
Not applicable.
The author declares no conflicts of interest.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
Alan Walters S. Horseradish pollination, sterility, and mate limitation. Crop Breed Genet Genom 2025;7(3):e250010. https://doi.org/10.20900/cbgg20250010.

Copyright © Hapres Co., Ltd. Privacy Policy | Terms and Conditions