Location: Home >> Detail
TOTAL VIEWS
Immunometabolism. 2020;2(4):e200030. https://doi.org/10.20900/immunometab20200030
1 Immunometabolism Research Group, Department of Systems Biology, Institute of Biomedical Sciences 1, University of São Paulo (ICB1-USP), São Paulo 05508-000, Brazil
2 Laboratory of Experimental Surgery, Department of Surgery, Clinics Hospital of the Faculty of Medicine, University of São Paulo (HC-FMUSP), São Paulo 01246-903, Brazil
* Correspondence: José Cesar Rosa Neto.
Background: White adipose tissue is an essential reservoir of energy that stores and releases fatty acids and secretes hormones, inflammatory cytokines and adipokines in health and cancer. The adipose tissue modulates cancer development and treatment, affecting responsiveness to chemotherapy, quality of life and survival. In addition, adipose tissue is damaged by doxorubicin, which is a non-selective anticancer drug widely used in clinical practice.
Aim: This review was focused on the relevance of the white adipose tissue and how it can be affected by doxorubicin and cancer, the mechanisms involved and possible co-therapies that improve white adipose tissue functions.
Scope of review: Adipose tissue complexity can influence cancer development, treatment and survival. The adipose tissue secretes adipokines that have paracrine and endocrine effects and may influence tumourigenesis, survival and quality of life in patients with cancer. The chemotherapeutic drug doxorubicin promotes deep impact on the adipose tissue, inhibiting adipogenesis and lipogenesis. Doxorubicin also causes downregulation on peroxisome proliferator-activated receptor gamma (PPARγ) and 5' adenosine monophosphate-AMP-activated protein kinase (AMPK) signalling in white adipose tissue, affecting lipid and glucose metabolism. Some alternative therapies, such as metformin, pioglitazone and physical exercise may contribute to mitigate side effects of doxorubicin.
Conclusion: White adipose tissue has a complex and intricate role on cancer and is deeply affected by doxorubicin leading to a deep impact on adipose tissue function and worse quality of life. Potential co-therapies to prevent the side effects of doxorubicin should be studied to improve the quality of life of doxorubicin-treated patients.
The adipose tissue is essential for metabolism; it releases fatty acids in some physiological situations such as starvation, exercise or sleeping. The main functions of the adipose tissue are to supply nutrients, secrete a large variety of hormones, inflammation factors, liposoluble vitamins and adipokines, which lead to various endocrine and paracrine effects [1,2]. Adipokines and lipids contribute to tumour environment, while stromal and immune cells provide inflammatory factors that affect tumour development and progression [3].
Excess or absence of white adipose tissue (WAT) can affect cancer growth in the host, modulate cancer treatment and quality of life in patients. While large adipose tissue may secrete inflammatory factors related to cancer development and impair the efficacy of chemotherapy by modifying adipokine and fatty acid composition [4], the loss of adiposity in patients with advanced cancer may contribute to mortality and even affect the response of chemotherapy against cancer [5,6]. Thus, low or high body mass are associated with elevated mortality after cancer diagnosis [6,7].
Doxorubicin is a non-selective chemotherapeutic drug widely used in clinical practice against many types of cancer such as lymphoma, lung, breast and ovarian cancer. Doxorubicin is derived from Streptomyces peucetius and is classified as an anthracycline [8]. There are three more anthracyclines, including daunorubicin, epirubicin and idarubicin, presenting differences in chemical structure, target and toxic properties [9]. The mechanisms of action of doxorubicin are based on cell death and cell growth arrest and promote apoptosis, necrosis and autophagy [10]. Doxorubicin promotes DNA intercalation, in which a complex is formed between the DNA double strand and the drug, causing breaks in DNA helix and leading to formation of fragmented nuclei. Doxorubicin blocks the action of topoisomerase 2, an essential enzyme for DNA replication, resulting in inhibition of cell proliferation and cell death. Then, it produces a semiquinone radical, that reacts with DNA, leading to oxidation of DNA by superoxide, hydroxyl and peroxide free radicals, which can also damage cell membranes through lipid peroxidation, triggering cell death pathways [10,11].
Doxorubicin is toxic to both cancerous and healthy cells, what limits its usage. Cardiotoxicity is the most studied side effect of doxorubicin, but it is also toxic to other organs such as the adipose tissue [12]. Doxorubicin induces body weight loss and adipose mass atrophy due to its effects on key factors of lipid and glucose metabolism, such as peroxisome proliferator-activated receptor gamma (PPARγ) and 5' adenosine monophosphate-AMP-activated protein kinase (AMPK), which results in inhibition of adipogenesis and lipogenesis [12,13]. Adipogenesis and lipogenesis are physiological processes in which fibroblasts-like progenitors cells turn into mature adipocytes and start to accumulate fat as lipid droplets [14]. The expansion of adipose tissue can contribute to metabolic health through the differentiation of the tissue into smaller adipocytes and avoiding the formation of large adipocytes, which can secrete pro-inflammatory cytokines and can also supply nutrients to nearby organs and protect against mechanical stress [14]. Thus, adipose tissue atrophy caused by toxic effects of doxorubicin can compromise metabolic health.
Loss of adiposity and skeletal muscle mass is a common syndrome observed in patients with cancer, involving increase in lipolysis, fatty acid oxidation and secretion of pro-inflammatory factors by adipose tissue, which are mechanisms related to cachexia [15,16]. Thus, co-therapies that mitigate the chemotherapy-induced fatty acid release into systemic circulation can contribute to better prognosis for patients with cancer. Therefore, this review was focused on how the complexity of adipose tissue can be affected by cancer and doxorubicin, mechanisms involved and possible alternative therapies that may mitigate the doxorubicin side effects on adipose tissue.
Adipose tissue is a non-fibre subtype of connective tissue, formed mainly by adipocytes and accompanied by a stromal vascular fraction, which consists of vascular endothelial cells, preadipocytes, fibroblasts, extracellular matrix and a mixture of immune cells [17]. In human body, there are four types of adipocytes: white, brown, beige and pink [18,19].
The white adipocytes have variable size and consist of unilocular lipid droplets, while brown adipocytes have numerous lipid droplets (multilocular) and high level of oxidative rate, high expression of uncoupling protein 1 (UCP-1) and are responsible for thermogenesis [20,21]. Furthermore, there are some other cells in the WAT that can express UCP-1 known as beige or brown-like adipocytes [22]. Basically, WAT and brown adipose tissue have opposite functions (storing and dissipating energy, respectively) and both are essential for survival [23]. Moreover, mice with high levels of brown adipocytes spread among WAT were less prone to develop obesity [24]. Finally, the pink adipocytes are white adipocytes located in the mammary gland that transdifferentiate during pregnancy and lactation into cells whose main function is milk secretion [25,26]. These cells have been recently named as pink adipocytes because of the macroscopic mammary gland colour during pregnancy [25,26].
In general, WAT depots are identified as subcutaneous or visceral and can be found in different areas of the body; the location and quantity of WAT are related to propensity to cardiovascular diseases [27]. Many studies [13,28,29] consider an increase in the mass of the visceral adipose tissue as being the trigger of insulin resistance and later, metabolic syndrome associated with low grade inflammation.
Plasticity is an important characteristic observed in adipose tissue due to its ability to proliferate, differentiate and transdifferentiate, which means that a mature adipocyte can become another cell type through a reversible process. For example, white-to-brown transdifferentiation may occur in case of chronic cold exposure, a process called browning [18]. Furthermore, adipose tissue can undergo remodelling, which occurs especially in the WAT in situations such as greater caloric consumption compared to daily energy expenditure, resulting in exacerbated accumulation of triacylglycerol in the adipocytes. As a consequence, WAT hypertrophy or hyperplasia may occur.
Beyond the adipocytes, the adipose tissue consists of resident and transient immune cells, including macrophages, mast cells, eosinophils, lymphocytes, dendritic cells, neutrophils and other stromal cells [3]. In humans, a study using immunohistochemistry techniques showed that the majority are of the immune cells in the adipose tissue are macrophages. Moreover, the proportion of macrophages can range from 4% in visceral fat of normal weight subjects up to 12% in obese patients yet these immune cells are responsible for secretion of most of the cytokines and maintenance of inflammation [30,31].
Macrophages founded in adipose tissue are in the majority derived from monocyte-derived macrophages, which are recruited to the adipose tissue based on high expression of monocyte chemoattractant protein-1 (MCP-1) [32]. Adipose tissue macrophages (ATMs) show plasticity and they can assume phenotypes that depend on the crosstalk with other infiltrated immune cells (lymphocytes, eosinophils and neutrophils) and with the adipocyte itself. In regards of the polarization of macrophages, the M1 macrophages (classical macrophages) show pro-inflammatory characteristic with tumouricidal and anti-bactericidal properties [32]. In contrast, M2 macrophages, or alternative activation, is associated with the resolution of inflammation [33]. M2 macrophage showed different subsets that have already been described: M2a, wound‐healing macrophages that minister tissue repair; M2b, characterised by immunoregulation, promotion of infection and tumour progression; M2c, macrophages with anti-inflammatory and phagocytic properties; and M2d, tumour-associated macrophages that promote tumour progression and angiogenesis [34]. It is interesting that the metabolism varies among macrophages phenotypes and is crucial to fate the polarization. While in M1 macrophages a glycolytic metabolism is predominant, as it is a faster way of producing energy the M2 shows more oxidative metabolism, using fatty acids as substrate [35].
Any switch on ATMs profile may lead to increased release of adipokines (by the adipose tissue) and cytokines (by the macrophages) associated with inflammation [33]. It is extremely important to highlight that immune cells of the adipose tissue not only include macrophages but also other myeloid and lymphoid cells. Mast cells, for example, have been indicated as mediators of macrophage infiltration due to faster increase in the number of macrophages upon their interaction with mast cells than that after the exposure of macrophages to high fat diet [36]. Moreover, dendritic cells play a role in the differentiation of pro-inflammatory Th17 cells, which results in polarisation of M1 macrophages [37].
One of the hypotheses that explain the inflammatory environment on obese that lead to increase on recruitment of immune cells to this tissue, is the reduction in oxygen supply due to adipocytes hypertrophy and subsequent restriction in blood flow [38]. This hypoxic microenvironment induces the activity of some transcriptional factors, such as hypoxia-inducing factor 1 alpha (HIF-1a) and drives the fibrotic and pro-inflammatory response, stimulating the chemotaxis of macrophages by secretion of type 1 monocyte chemoattractant protein (MCP-1) [38]. As a consequence of the increase in ATMs and their subsequent inflammatory response, a state of chronic low grade inflammation, characterised by predominant production/secretion of pro-inflammatory cytokines, is trigged [39]. The low grade inflammation is an important risk factor to tumourigenesis and to sustain tumour growth [40].
The WAT is located throughout the human body contributing as a connective tissue between organs and providing mechanical protection [41]. The anatomical distribution and localisation may be important to maintain the homeostasis, for example, lipids depots near reproductive system can support spermatogenesis in mice [42], suggesting they may be an in situ nutritive or trophic factor.
Fat composition in the human body tends to be stable, besides that, the amount of adipose tissue is modulated by many factors, such as internal stimuli including gender, age, ethnicity, diseases, hormones and use of medicaments and external stimuli including climate, stress, diet and physical activity [1,43].
Some epidemiological studies have found that obese and overweight individuals have an augmented risk for some types of cancer and mortality [44]. Prospectively, Calle et al. (2003), after analysing 900,000 north-Americans, showed that elevated body mass index (BMI) is associated with elevated rate of death from some types of cancer with 14% of all deaths for men and 20% for women [45]. Excess of adiposity, common in obesity, is generally a risk factor for several types of cancer, including colorectal [46], pancreatic, bladder, renal, ovarian, brain [47] and breast [48], since the excess of fatty acids can trigger tumorigenesis [49,50]. On the other hand, in some types of tumour, for instance the colon cancer, low BMI is associated with increased progression and death [51]. Besides epidemiological data, in clinical practice it is essential to manage body weight in cancer patients and evaluate individual variability, cancer stage and type of cancer.
As any other endocrine organ, in order to maintain homeostasis, WAT secretes specific adipokines depending on the stimulus. Caloric deficits or fat accumulation are common reasons for production of adipokines by the adipose tissue; however, other situations must be considered, such as exercise and its variations (duration, intensity, etc.) and metabolic changes in microenvironments such as cancer [19]. The crosstalk between the adipose tissue and carcinomas, especially WAT, which is linked to a high cancer risk, will be further discussed.
Leptin is an adipokine that regulates feeding behaviour and energy expenditure and it is found in high concentrations in obese individuals; however, they seem to be resistant to this adipokine. Conversely, leptin activates pro-inflammatory cytokines secretion from monocytes and macrophages [52]. Adiponectin is another adipokine mainly secreted by adipocytes, it has been shown to be a potent target for glucose uptake through AMPK in the skeletal muscle [53] and for avoiding LPS-induced secretion of pro-inflammatory cytokines by macrophages via inhibition of NF-κB [54].
Adipose tissue and immune cells provide a suitable microenvironment for tumour development and progression (Figure 1) by trigger and support low grade inflammation [3]. More recently, the link between adipose tissue and tumour interaction was revealed, indicating that white adipocytes may play a role in cancer development [55]. It was demonstrated that a strong communication exists between white adipocytes and breast cancer cells, called cancer-associated adipocytes. These adipocytes are able to secrete high quantities of chemokines responsible for cancer progression, such as tumour necrosis factor alpha (TNF-α), vascular endothelial growth factor (VEGF) and proteases that promote breast cancer aggressiveness [56].
The crosstalk between adipose tissue and cancer is even more concerning due to the augmented VEGF expression that leads to angiogenesis, particularly in the visceral WAT [57], consequently providing all the factors that tumours need to support their metabolic activity. In theory, pro-inflammatory tumour microenvironment would be suitable for M1 macrophages; however, recruited monocytes are more prone to the M2 profile [58]. The pro-tumour macrophage polarisation (M2) seems to be mediated by high lactate exposure (the main metabolite from the tumoral aerobic glycolytic pathway) [59]. Notably, this shift in immune cell profile is one of the targets of cancer immunotherapies, explaining why identifying biomarkers is relevant for improving sensitivity of therapies and/or diminishing cancer cell resistance [60].
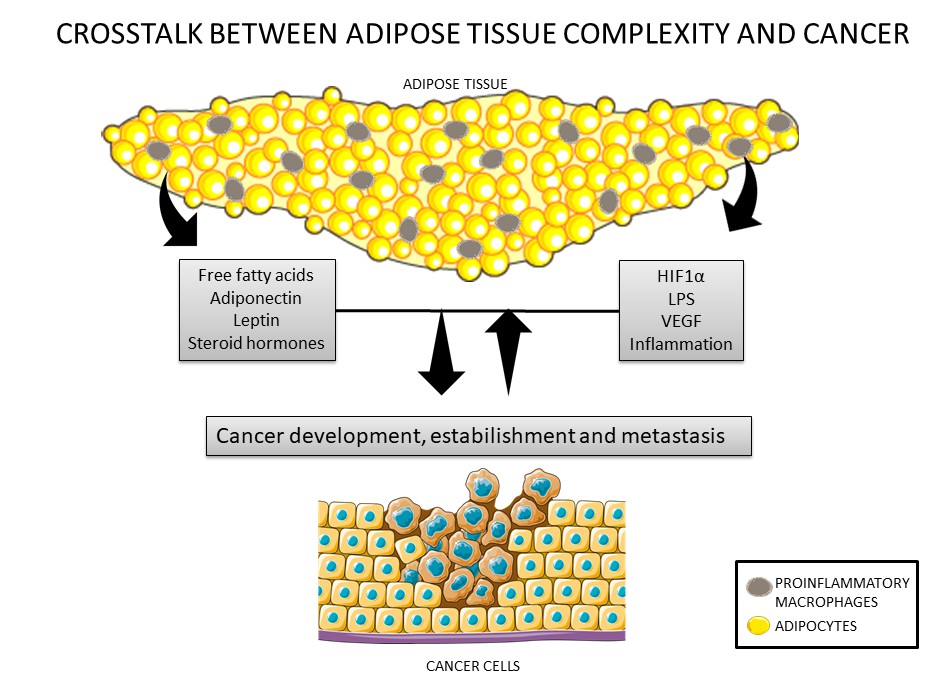 Figure 1. Crosstalk between cancer cells and the adipose tissue. Adipose tissue secretes adipokines such as leptin, adiponectin, inflammatory factors, steroid hormones, and nutrients that can modulate cancer development and establishment. In addition, cancer cells can also secrete inflammatory factors that can lead to modulations in adipose tissue, such as recruitment of macrophages.
Figure 1. Crosstalk between cancer cells and the adipose tissue. Adipose tissue secretes adipokines such as leptin, adiponectin, inflammatory factors, steroid hormones, and nutrients that can modulate cancer development and establishment. In addition, cancer cells can also secrete inflammatory factors that can lead to modulations in adipose tissue, such as recruitment of macrophages.
Therefore, besides the low grade inflammation founded in obesity another risk factor for tumourigenesis is insulin resistance that results in increased growth factors such as insulin growth factor (IGF-1). Hyperinsulinemia promotes IGF-1 secretion, leading to enhanced mitosis, angiogenesis, and apoptosis inhibition; thus, hyperinsulinemia, together with pro-inflammatory cytokines secreted by the adipose tissue in obesity favours tumour progression [61]. Thus, diseases related to insulin resistance, such as obesity and diabetes, contribute to high risk of development of some types of cancer [62].
Besides adipokines and cytokines, adipose tissue is important to regulation of sexual hormones and cortisol by expression of enzymes such as p450 Aromatase, 11β-Hydroxysteroid dehydrogenase 1 (HSD1) and 17β-HSD Aromatase, which produce extragonadal steroid hormones. These enzymes, respectively, can promote the conversion of androgen into oestrogen, estrone in estradiol and cortisone into active cortisol [63]. Aromatase activity promotes breast tissue proliferation through the release of endogenous oestrogen, causing an increase in the risk of breast cancer in postmenopausal women [64].
Furthermore, alterations in adipose tissue derived hormones subsequently lead to local and systemic effects in glucose homeostasis and can contribute to tumour microenvironment, since the tumour cells show elevated energetic metabolism and they use large amounts of glucose. It is well known that the primary source of energy in cancer cells is glucose, thus, high glucose levels in the systemic circulation can increase the risk of cancer cell growth and survival [62].
Despite of obesity has been related to elevated risk for death, compared to normal-weight [65], the weight loss is also a poor prognostic sign and often is considered a marker of more aggressive or advanced cancer. Effects of cancer on metabolism can lead to cachexia, a syndrome characterised by significant reduction of muscle mass with or without reduction of fat mass [66,67]. It has been shown that loss of adipose tissue is a result of alterations in lipid uptake, lipogenesis and lipolysis, which can worsen the cancer treatment responsivity [68]. Weight loss is an indicator of reduced survival for patients with advanced cancer, loss of adipose tissue is faster than loss of lean mass [69]. Significant weight loss in lung cancer may lead to hyperlipidemia and insulin resistance, which can be explained by factors such as anorexia, loss of appetite, cytokines production, macrophage infiltration, adipocyte dysfunction and fibrosis [69]. For gastrointestinal cancer, weight loss pre- and during chemotherapy is associated to poor survival [70]. In summary, the excess or loss of WAT can be a trigger for cancer related morbidity and mortality; in this context, it is essential to consider the individual variability and cancer stage (diagnosis, treatment and prognosis).
In cancer, the elevated secretion of cytokines by tumour cells can activate molecular pathways of lipolysis, such as activation of protein kinase A (PKA) [71]. Tumour secretion of interleukin 6 (IL6) and TNF-α leads to an increase in the rate of lipolysis and can contribute to metabolic dysfunction in tumour and adipocytes cells [72]. Then, free fatty acids (FFA) induce autocrine and paracrine signalling, increasing TLR-4 pathway and consequently increased the expression of inflammatory cytokines [72].
FFA can be stored into lipid droplets, which are organelles existent mainly in adipocytes; however, lipids also can be stored in non-adipocyte cells, such as in liver, heart, kidney, skeletal muscle and even cancer cells, additionally excess of FFA can damage the functions of these cells, a process called lipotoxicity [73,74]. Then, inflammatory response can also contribute to lipotoxicity, inflammatory pathways can induce elevation of FFA into systemic circulation by increasing on lipolysis and blocking the PPARγ, leading to reduction on adipogenesis and fatty acid uptake, whereas PPARγ regulates the transcription of CD-36 and lipoprotein lipase (LPL) [73,74].
Colon adenocarcinoma has been associated with high FFA levels into systemic circulation and high LPL activity in the adipose tissue and heart [75]. The main function of LPL is to uptake FFA from the lipoproteins, which favours the accumulation of fatty acids into lipid droplets [74,75]. Inhibiting the fatty acid transporter CD-36 and stearoyl-CoA desaturase 1 (SCD1) in breast cancer cells leads to attenuation of cell growth. SCD-1 catalyses the conversion of saturated fatty acids into monounsaturated fatty acids and CD-36 transports this fatty acid, modulating membrane composition, fluidity and others second messengers. CD-36 and SCD-1 can be overexpressed in other cancer cells such as lung, colon and renal carcinoma [76].
Besides, many types of human solid tumours consist of lipid droplets composed by cholesterol and triacylglycerol, which are used as an energy source by neoplastic cells [77,78]. Tumour cells utilise fatty acid as source of energy to maintain their development and lipids are essential components for cell membranes and some organelles. During cell division, the tumour cells begin cholesterol biosynthesis before DNA duplication, showing that fatty acid synthesis is essential for cell proliferation [79]. The tumour lipid droplets compensate for lower nutrient supply and oxygen availability in the tumour microenvironment, thereby supporting redox homeostasis and membrane biogenesis during the rapid cell growth and tumourigenesis [74].
In addition, cholesterol and phospholipids are components of cellular membrane that contribute to properties such as fluidity and rigidity, modulating uptake of nutrients, hormones and vitamins [79]. Hilvo et al. (2014) found that breast cancer cells can express high levels of membrane phospholipids including phosphatidylcholines, sphingomyelins and ceramides [80]. Cholesterol-lowering medications can lead to cancer cell apoptosis and cell cycle arrest mainly in colorectal cancer [79,81–83]. Statins can impact metastasis and invasiveness properties of cancer cells through inhibition of Ras, which is frequently mutated in some neoplastic cells, Rho and activation of caspase 9 [83]. Therefore, many anticancer drugs affect lipid metabolism in cancer cells by modulating cholesterol production and inhibiting fatty acid synthetase and ceramide production, showing that lipid metabolism can be a target for therapies based on control of cancer cells division [79].
Doxorubicin is a well-known chemotherapy drug, which is widely used for treatment of solid tumours such as breast, liver, stomach, prostate, ovarian and lung cancer and soft tissue sarcomas [84]. Doxorubicin is one of the most potent anticancer drugs; it is an anthracycline drug that can be prescribed alone or in combination with others [84]. Cancer cells have a highly competent cell machinery, which allows them to establish and develop themselves in the host [85], for this reason, non-selective chemotherapy and radiotherapy can be used to treat the patient with cancer; however, the effects of these treatments are toxic to several types of cells such as adipocytes, myocytes and cardiomyocytes [85]. In this section, will be described recent studies focused on the effects of doxorubicin on WAT.
Our group showed that a single dose of doxorubicin (15 mg/kg body weight) caused rapid loss of adipose mass and inhibited adipogenesis and lipogenesis with an imbalance in adipokines, clearly showing that doxorubicin affects negatively the WAT function [12]. Doxorubicin impaired adipogenesis through downregulation of PPARγ, CAAT enhancer-binding protein alpha (C/EBPα) and sterol regulatory element-binding protein 1c (SREBP1c) that are key transcription factors for adipocyte development [12]. Besides, doxorubicin compromised lipogenesis, reducing the incorporation of fatty acid into triacylglycerol in retroperitoneal adipose tissue, reducing the expression of lipogenic enzymes such as fatty acid synthase (FAS) and acetyl-Coa carbolyxase (ACC) together with the inhibition of lipid droplets in 3T3L1 cells [12].
In addition, doxorubicin elevates lipolysis through upregulation of the enzyme adipose triglycerides lipase (ATGL), elevating lipid profile into systemic circulation [86]. Vergoni et al. (2016) showed that a single injection of doxorubicin elevated FFA levels into systemic circulation [87]. In contrast, in our study in vitro, lipolysis and ATGL were inhibited by doxorubicin [88].
The result of impaired lipogenesis and augmented lipid profile can alter adipose tissue functions and glucose metabolism [86]. Doxorubicin reduced adiponectin content in WAT and its gene expression [87,88]. Adiponectin regulates lipid and glucose metabolism and doxorubicin treatment decreased this adipokine concentration and lowered glucose uptake after insulin stimulus in mice and 3T3L1 cells [12].
Moreover, doxorubicin lead to inflammation and fibrosis on WAT. A single dose of doxorubicin promoted the increased on expression of inflammatory cytokines (TNF-α, IL6 and interleukin 1 beta (IL1β) concomitantly with raised infiltration of macrophages [89]. Moreover, it was observed the presence of fibrosis can damage expandability of subcutaneous adipose tissue mediated by the high extracellular matrix rigidity, leading to impairment in metabolic pathways [88].
Besides, it was demonstrated that obese tumour-bearing mice presented reduction on tumour cells death by doxorubicin via alterations in lipid profile markers and fatty acid composition [4], showing that doxorubicin can affect the crosstalk between adipose tissue and cancer cells [89].
Therefore, the main molecular mechanism that link the effect of doxorubicin with disturbing on glucose and lipid metabolism is the impairment of PPAR-γ and AMPK signalling on WAT (Figure 2). For this reason, PPARγ and AMPK and their functions are detailed in the next sections.
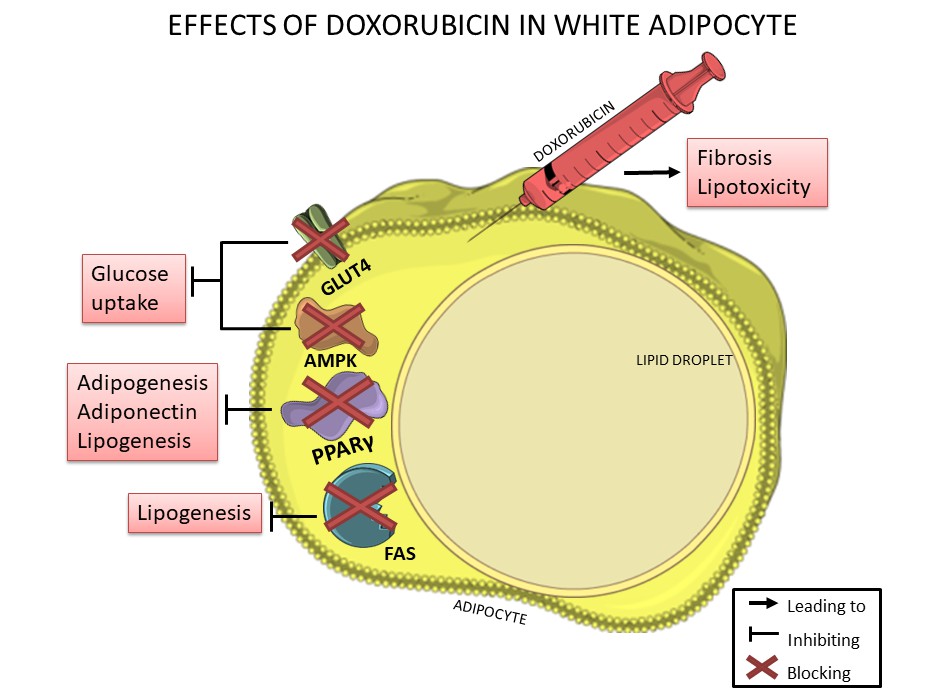 Figure 2. Doxorubicin negatively affects white adipocytes, reducing adipogenesis, lipogenesis, adiponectin production and glucose uptake thorough downregulation of PPARγ, FAS, GLUT-4 and AMPK, which are essential for lipid and glucose metabolism. In addition, doxorubicin can induce fibrosis in adipose tissue and can also induce lipotoxicity.
Figure 2. Doxorubicin negatively affects white adipocytes, reducing adipogenesis, lipogenesis, adiponectin production and glucose uptake thorough downregulation of PPARγ, FAS, GLUT-4 and AMPK, which are essential for lipid and glucose metabolism. In addition, doxorubicin can induce fibrosis in adipose tissue and can also induce lipotoxicity.
In adipocytes, PPARγ is downregulated by doxorubicin [12], PPARγ plays an important role in the differentiation into mature adipocytes, adiponectin production, lipid metabolism and inflammatory pathway [90]. PPARγ is a nuclear receptor that forms a heterodimer with the retinoid X receptor and binds to the peroxisome proliferator response element gene promoter, resulting in the regulation of gene transcription mainly involved in lipid and glucose metabolism [91].
PPARγ is considered the master regulator of adipogenesis [92]. In vitro non-adipocytes cells can be stimulated to differentiate into mature adipocytes by allowing them to express PPARγ mRNA and then start to accumulate lipid droplets [92]. PPARγ is essential for adipocytes maturation and survival as it is observed in conditional fat specific knockout model once total knockout models are lethal [92,93].
PPARγ is necessary for the regulation of insulin sensitivity and for this reason it is the pharmacologic target to treat patients with insulin resistance. The effects of PPARγ agonists are explained by increase glucose uptake and adiponectin secretion by adipose tissue, decreasing FFA into systemic circulation and reducing pro-inflammatory cytokines production [92]. However, as previously mentioned, PPARγ is downregulated by doxorubicin damaging in vitro differentiation into mature adipocytes, reducing glucose uptake and adiponectin concentrations in vivo [12].
Moreover, PPARγ is detected in most tissues and also expressed in tumour, intestine and immune system [94]. In some types of cancer, PPARγ has shown antitumour effects [95]. Recently, a correlation between PPARγ and P-gp has been suggested. P-gp is a target of Wnt/β-catenin pathway, an essential pathway on epithelial to mesenchymal transformation (EMT) and its mRNA downregulation is a consequence of reduction in β-catenin levels caused by PPAR agonists [96].
In addition, PPARγ can act on chemotherapy sensitivity. When activated, PPARγ was efficient in reversing the sensitivity of cancer cells in combination with doxorubicin [97]. Thus, the expression of PPARγ has been associated with greater survival in patients with colorectal cancer, implicating that chemotherapeutic sensitivity would be dependent on PPARγ expression in the tumour [98].
The mechanisms by which PPARγ agonists have been considered potential adjuvants in conventional therapies are angiogenesis, inhibition of cell proliferation and apoptosis and chemoresistance [98,99]. A study conducted by Patel et al. (2001) showed that PPARγ activation increased the expression of a potent tumour suppressor, phosphatase and tensin homolog, in both colon and breast cancer cells, which reduced their rate of proliferation [100].
AMPKDoxorubicin can reduce AMPK expression in adipose tissue (Figure 2) and even in other tissues such as skeletal muscle and heart [12,101,102]. Nonetheless AMPK is a regulator of multiple metabolic pathways and several studies have shown its activation importance for treating insulin resistance, diabetes, obesity, cardiovascular disease, non-alcoholic fatty liver disease and cancer [103–107].
AMPK is a serine/threonine-specific protein kinase that exists as multiple heterotrimeric complexes comprised of a catalytic α subunit (α1 and α2), a regulatory β subunit (β1 and β2) and γ (γ1, γ2, γ3) subunits [105]. These subunit conformations are uniquely distributed across different cell types, white adipocytes expresses AMPK complexes composed predominantly by α1, β1, β2, γ1 and γ2 subunits [108].
Several pathways of glucose and lipid metabolism in WAT have been demonstrated to be potently regulated by AMPK. The activation of AMPK in WAT is noted under conditions of increased β-adrenergic stimulation, which occurs during fasting and physical exercise, leading to rapid adjustments in the metabolism of substrates [109,110].
Some metabolic responses induced by the activation of AMPK in adipocytes are quite different from those observed in skeletal muscle cells and hepatocytes. This indicates that AMPK plays its role as a cell energy sensor in a time-dependent and tissue-specific manner [108]. Additionally, effects of AMPK on the adipocytes metabolism may vary depending on the duration of AMPK activation [109,110] and AMPK can alter long term changes in WAT function, positively regulating the expression of genes (PGC-1α, PPARγ/α/δ, CPT-1b and COX) that markedly increase oxidation, remodelling or metabolism of adipocytes [108].
Furthermore, AMPK disruption is common in cancer associated to cachexia and AMPK activation in cachectic mice can reduce WAT wasting [111]. Hence, strategies to prevent AMPK and PPARγ dysfunction can be alternative co-treatments for chemotherapy.
It is necessary to study co-therapies that mitigate side effects promoted by doxorubicin and others treatments against cancer, focusing in quality and life expectancy improvements of oncologic patients. Thus, we propose that PPARγ and AMPK are two molecular pathways that can improve outcome in different cancer types with benefits on whole body homeostasis, for this reason, will be briefly discussed the therapies with metformin, pioglitazone and physical exercise as potential co-treatments to prevent doxorubicin-induced disturbs in cancer patients.
MetforminMetformin is a classical anti-diabetes drug that reduces hyperglycaemia and cardiovascular risk, induces weight loss and improves insulin resistance [112,113]. Molecular mechanisms of metformin are associated with AMPK activation, whose function is well known in liver and muscle cells [114]. Additionally, metformin is able to increase AMPK activity in adipocytes [115].
Many studies have shown the use of metformin as an anticancer/anti-tumour agent individually or in combination with frequently used chemotherapeutic agents [116,117]. Diabetic individuals on metformin treatment have a lower risk of developing cancers than non-treated diabetics [116,117].
Furthermore, diabetic individuals with cancer who are treated with metformin show a positive response to chemotherapy treatment and have high survival rates and a better prognosis when compared to individuals who did not use metformin [116,118]. In addition to these classic effects of metformin, it also shows positive effects when used in conjunction with chemotherapy drugs, more specifically those from the anthracycline family (doxorubicin and daunorubicin), showing reduced growth and survival of lymphoma cells, T-acute lymphoblastic leukaemia cells and acute lymphoblastic leukaemia [119–123].
Metformin prevented fibrosis and restored glucose uptake in subcutaneous adipose tissue after insulin stimulation in mice treated with doxorubicin, yet the drug was unable to prevent other side effects, such as loss of adipose tissue and inflammatory response [88]. Subcutaneous adipose tissue from metformin-treated mice also showed a reduction in collagen deposition and reducing fibrosis [88].
Moreover, metformin can contribute to reduction in the dosage of doxorubicin necessary to prolong remission and consequently, can reduce the cardiac toxicity of anthracyclines [122]. In general, metformin can promote protective effects to patients during chemotherapy.
PioglitazonePioglitazone is an antihyperglycaemic drug, together with metformin they are considered safe and for this reason mostly prescribed for patients with diabetes mellitus [124]. Pioglitazone is a well-known PPARγ agonist, classified in the family of thiazolidinediones, it modulates insulin sensitivity through improvement in β-pancreatic cells, liver, skeletal muscle and WAT [125]. This drug also enhances PPARγ gene expression leading to upregulation of adiponectin secretion by WAT resulting in glycemic homeostasis and contributing to adipocyte functions [124].
In addition, pioglitazone can exert anti-cancer effects through apoptosis induction and cell cycle arrest leading to decreased tumour incidence in chemically-induced lung and colon cancer in animals [124,126].
Furthermore, pioglitazone can attenuate doxorubicin-induced chemoresistance and side effects. Cancer cells can present doxorubicin-induced chemoresistance, causing elevation of P-gp gene expression and then pumping out the chemotherapy drug to extracellular fluid. Pioglitazone may be an alternative therapy to avoid chemoresistance reducing P-gp expression in osteosarcoma cells [97]. Pioglitazone also protect kidney from toxicity doxorubicin-induced, attenuating fibrosis and inflammatory pathways [127].
Besides that, most studies with pioglitazone as chemoprotective drug are experimental models and they are not related to WAT, for this reason, it is suggested that pioglitazone is an alternative therapy against side effects induced by doxorubicin.
Physical ExerciseSedentary lifestyle can be considered a disease. Regular physical exercise is an excellent tool to prevent chronic diseases, such as diabetes, cardiovascular diseases and obesity, yet, exercise is a recommended strategy for prevention and treatment of some types of cancer [128,129].
Physical exercise also plays an important role in the rehabilitation process in patients with cancer [130,131]. Physical exercise post and during cancer treatment is safe and induces the positive effects on muscular strength, mental health and cardiorespiratory fitness, as well showed by a systematic review and meta-analysis [131]. This supports the conclusion that physical training is safe during and after cancer treatment and can improve functional capacity, quality of life and reduce cancer related fatigue in various groups of cancer survivors [130–133]. In addition, Lira et al. (2008) showed that aerobic exercise promotes a protective effect, reducing 10 times the tumour weight in Walker-256 tumour-bearing rats [134].
Some chemotherapies are related to sarcopenia, loss of adipose mass, compromised quality of life, cardiotoxicity and asthenia [13,135–138], however, few studies have showed the effect of physical exercise in adipose tissue during chemotherapy treatment. Physical exercise consistently improves the quality of life by inducing significant alterations in body composition, metabolism and chronic inflammation. Moreover, regular physical exercise has been reported to be an inducer of anti-inflammatory response. Studies have shown that exercise-mediated anti-inflammatory effect leads to improved protection against chronic inflammatory conditions and levels of pro-inflammatory cytokines and C-reactive proteins [139–142].
Physical exercise requires an increase in muscle contractions, which initiates increased production and release of numerous muscle-derived cytokines and other proteins called myokines; based on this process, the skeletal muscle is defined as an immunogenic secretory organ [143]. Among these myokines, the role of IL6 in the metabolism has been well studied and described. The effects of IL6 form a great paradox; in infections and chronic diseases, the IL6 acts as a phase acute protein and a pro-inflammatory cytokine, whereas the IL6 released from skeletal muscle contraction shows anti-inflammatory properties and anti-tumoural effects [144,145]. Moreover, IL-6 recombinant infusion induces insulin-mediated glucose uptake and improvement on fatty acid metabolism that should be dependent of AMPK signalling [146]. Different research groups have tried to clarify why these different effects on IL6 are dependent on the stimulus and site of production. Until this moment is most acceptable answer is that the acute and transient substantial IL6 increase into systemic circulation after exercise induces the beneficial effects, while the chronic but less intense increase induces the deleterious effects. In this sense, it is observed a relation between high IL-6 and poor prognostic in diseases, short life expectancy, increased tumour size, proteolysis and persistent inflammation. Thus, new studies are necessary to elucidate the difference between the molecular pathways and the good and bad effects of IL6.
Exercise leads to the activation of AMPK in the WAT [109] concomitantly with adrenergic stimulus, from which it can be predicted that β-adrenergic agonists and their second messenger cAMP stimulate AMPK activity [110,147]. Moreover, IL6 can activate AMPK in muscle and adipose tissue, which contributes to the increase in AMPK activity in these tissues in response to exercise. Kelly et al. (2004) also suggested that a genetic lack of IL6 is associated with a decrease in AMPK activity [148].
Overall, physical exercise can promote a protective effect on the maintenance of the anti-inflammatory profile in adipose tissue in tumour-bearing rats [149] and can mitigate the metabolic disturbance caused by tumour [150]; however, the protective role of exercise in chemotherapy treatment in patients with cancer is unclear.
The white adipose tissue has a complex and intricate role on sustained tumourigenesis, survival and quality of life in patients with cancer. Adipokines effects may influence cancer development and chemotherapeutic treatment. The chemotherapeutic drug doxorubicin disturbs physiological and immunometabolic functions of white adipose tissue, affecting lipid and glucose metabolism through disruptions on PPARγ and AMPK pathways. Hence, the study of potential co-therapies focused in these pathways, such as metformin, pioglitazone and physical exercise, can contribute to the attenuation of doxorubicin-induced side effects and can promote protective effects on white adipose tissue, consequently improving quality of life of doxorubicin-treated patients.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be considered as a potential conflict of interest.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
37.
38.
39.
40.
41.
42.
43.
44.
45.
46.
47.
48.
49.
50.
51.
52.
53.
54.
55.
56.
57.
58.
59.
60.
61.
62.
63.
64.
65.
66.
67.
68.
69.
70.
71.
72.
73.
74.
75.
76.
77.
78.
79.
80.
81.
82.
83.
84.
85.
86.
87.
88.
89.
90.
91.
92.
93.
94.
95.
96.
97.
98.
99.
100.
101.
102.
103.
104.
105.
106.
107.
108.
109.
110.
111.
112.
113.
114.
115.
116.
117.
118.
119.
120.
121.
122.
123.
124.
125.
126.
127.
128.
129.
130.
131.
132.
133.
134.
135.
136.
137.
138.
139.
140.
141.
142.
143.
144.
145.
146.
147.
148.
149.
150.
Biondo LA, Silveira LS, Teixeira AAS, Rosa Neto JC. White Adipose Tissue and Cancer: Impacts of Doxorubicin and Potential Co-Therapies. Immunometabolism. 2020;2(4):e200030. https://doi.org/10.20900/immunometab20200030

Copyright © 2020 Hapres Co., Ltd. Privacy Policy | Terms and Conditions