Location: Home >> Detail
TOTAL VIEWS
J Sustain Res. 2024;6(2):e240036. https://doi.org/10.20900/jsr20240036
1 Centro Conjunto de Investigación en Química Sustentable UAEMex-UNAM, Carretera Toluca Atlacomulco, Km 14.5, Toluca 50200, Mexico
2 Instituto de Química, Universidad Nacional Autónoma de México, Circuito Exterior, Ciudad Universitaria, Ciudad de México 04510, Mexico
3 Facultad de Ingeniería, Universidad Autónoma del Estado de México, Cerro de Coatepec S/N, Toluca 50100, Mexico
* Correspondence: Carlos E. Barrera Diaz.
Solar distillation is a highly interesting process due to its utilization of one of Earth's most abundant natural resources: solar energy. Recent research has delved into various geometries and modifications to enhance the efficiency of solar stills and optimize the solar energy capture in the basin. This work proposes a modification to the condensation cover, featuring a solar still design with a cylindrical basin and a concave condensation cover, where distillate collection occurs in the lower central part of the basin. The study evaluated two construction materials for the cylindrical basin, namely stainless steel, and aluminum with Teflon coating, in the distillation of synthetic polluted water (distilled water with Allura red dye at a concentration of 50 ppm). Experiments were conducted under the environmental conditions of Toluca, Mexico. Distinct variations in water and inner air temperatures were observed, attributed to the reduction in the total volume of the proposed solar still. Under optimal conditions, water temperatures reached a maximum range between 68 to 74 °C. The solar still with an aluminum basin featuring Teflon coating showed the highest efficiency in distilling Allura Red dye, as assessed by UV-Vis spectroscopy. Evaluation of water quality parameters (pH, electrolytic conductivity, total solids, and TOC) aligned with Mexican regulations, indicating that the proposed design is effective.
TOC, total organic carbon; CS, concentrator solar; SS, solar still; EC, emerging contaminants; BOD, biochemical oxygen demand; SSCC-SS, device with stainless steel; SSCC-AT, device with aluminum with Teflon coating
The rapid population growth, ineffective water resource management, and escalating contamination of existing freshwater reservoirs have collectively led to an inadequacy in meeting the long-term needs of the population [1]. Globally, as well as in Mexico, water is recognized as a pivotal element for social and economic development. The assurance of its quality stands as a key objective within the United Nations' sustainable development goals. Consequently, reducing pollution and enhancing wastewater management present opportunities to augment the secure reuse of this resource, thereby addressing issues of scarcity [2].
In recent years, the issue of emerging contaminants (EC) has attracted attention due to the possible adverse impacts on the environment. EC are chemical compounds of anthropogenic origin that are present in the environment; the main source of them is wastewater from industry or municipal wastewater, and it is due to their inadequate or non-existent treatment. They are usually present in complex matrices in very low concentrations and are classified according to their use such as pharmaceuticals, personal care, agriculture, as well as endocrine disruptors and microplastics, among others [3,4]. One of the emerging contaminants that has generated interest are dyes, since their disposal in the environment contaminates water bodies, consequently affecting their quality, aquatic life, and human health [5]. Most of the dyes used in the food and pharmaceutical industries belong to the group of azo compounds (Tartrazine, Sunset Yellow FCF, Azo Rubine, amaranth, Ponceau 4R, Allura Red AC, Brilliant Black BN, Brown HT are some examples); these are synthetic dyes and are designed to resist sunlight, oxidizing agents, soap, among other factors, which is why they have a long half-life that makes them difficult to degrade. Consequently, their presence has been reported in wastewater industrial effluents [6–8]. One of its main repercussions is that it increases the level of Biochemical Oxygen Demand (BOD) and reduces light penetration, which can cause drastic changes in aquatic life [9].
Hence, technologies have been developed for the degradation of persistent compounds in water, which have been applied for the degradation of azo dyes, such as chemical precipitation, microbial degradation, membrane separation, adsorption, photocatalysis, ozonation and electrochemical methods. However, those technologies require high cost of equipment, long processing time, generation of toxic intermediates, or high energy consumption [10,11]. In this sense, the use of solar energy for the separation of contaminants in water has been proposed for low-income countries. Solar energy is a stable, abundant, and universal source of renewable energy with high potential for its use; evaporation based on solar technology to purify contaminated water and has become a method of interest [12].
Solar distillation has enabled a variety of potential applications such as seawater desalination (the most common application), contaminated water purification, electrical power generation, and steam sterilization [13]. Solar distillation can become a useful process in rural and remote areas suffering from an acute water crisis due to contamination of groundwater and surface reserves, either due to natural or anthropogenic reasons [14]. However, it is a low-performance technology, which is why in recent years numerous authors have designed, evaluated, and improved conventional solar stills (single slope, double slope, among others), which differ in the geometry of the basin (a basin, multi-basin, stepped, spherical or hemispherical, V-shaped, tubular, etc.) and collection system. Similarly, the use and advancement of energy-efficient materials or materials endowed with internal energy storage capabilities, interface systems, solar concentrators, and reflective mirrors, among other innovations, play a significant role in enhancing the production efficiency of the solar distillation system [15–17].
Designs such as that of Arunkumar et al. [18] in which they implemented the cooling of the hemispherical lid through a constant flow of water over it, improved productivity from 34% to 42%. Attia, Zayed, et al. [19] made a comparison of the use of iron trays and V-corrugated iron trays with wick materials vs. a conventional hemispherical distiller, in which productivity increased from 33.94% to 61.67%. Other example of geometry such as the tubular solar still, Essa et al. [20] integrates and compare a rotating hollow drum and a rotating closed drum with an improvement of 140% and 175%. Hilarydoss et al. [21] evaluate a solar still with an inclined felt sheet in which they reach a productivity of 4.6 L/day on clear days. In conventional stills (single or double slope), materials have also been developed to improve heat storage and transfer, The use of a phase change material (paraffin wax mixed with Al2O3 nanoparticles) with an efficiency of 62.4% was obtained compared to 30% with a conventional one [22]. The use of jute fabric knitted and sand in whose improvement, they reached a productivity of 56.14% [23]. Additionally, the use of a system composed of graphite blocks as heat storage, internal solar reflectors, a fan, and a cold and hot water supply module to the condenser, in which they obtained a daily production of 2450 mL [24]. Kaviti et al. [25] utilized camphor-soothed banana stems to collect solar thermal energy and to transfer it to the water, resulting in an output of 934 mL/day. Suraparaju et al. [26] enhanced the desalination percentage by 29.67% using dried pond fibres (Algal Fibres). Kaviti, et al [27] evaluated a hybrid nanofluid of cerium oxide (CeO2) nanoparticles and multi-walled carbon nanotubes (MWCNTs) in a ratio of 80:20 with an output of 1430 mL per day. Kaviti et al. [28] optimized the efficiency of solar desalination by using a floating absorber (Cr-Mn-Fe oxide nanocoating) to stop the heat conducting to the condensed water, with a productivity of 390 mL/day. Kaviti and collaborators [29] coupled the basin liner with copper tubes and parabolic fins, with productivity of 4.86 L/m2 per day. From the above literature, it is observed that many studies investigate the energy storage and transfer for better efficiency. The maximum temperatures achieved for water range between 59 °C and 72.5 °C, with efficiencies between 40% and 69%, the designs are mainly focused on maintaining the temperature high (over 50 °C) as long as possible to promote constant condensation. However, there are limitations which hinder adoption of some of these technologies which require additional economic and specialized work investment specially in solar radiation-abundant remote or rural locations. It is important to mention that there are no works that report solar still with modifications in the geometry of the cover. In this aspect, the primary intention of the present research is to develop an easy manufacturing and low cost solar still using accessible accessories and implementing them in a concave cover following the basic operating principles of solar distillation.
The operating principle of a conventional solar distillation system is represented in Figure 1. It consists of a single slope solar still, normally constructed of fiberglass and covered with transparent glass. The basin is generally coated in black to absorb the greatest amount of solar radiation. The distiller faces south and the angle of inclination of the glass cover corresponds to the latitude of the place where it will be installed. The incident solar radiation is transmitted through the glass cover and is absorbed by the basin, most of the radiation is absorbed in the water. The heat gained inside the still is used to heat the water, which subsequently evaporates and condenses on the surface of the glass due to the difference in temperatures between the water and the glass. The water droplets descend into the collector by gravity and are collected in an external container. Distillers are generally sealed to minimize vapor leakage into the environment. The evaporation process in traditional distillers is relatively slow due to the influence of various parameters. The parameters studied include the intensity of solar radiation, ambient temperature, wind speed, solar geometric parameters (angle of latitude of the place, angle of declination), the inclination angle of the condensation cover, the basin absorptivity, thermal insulation, type of feed water, duration of daily sunlight and water depth in the basin, among others [16,17].
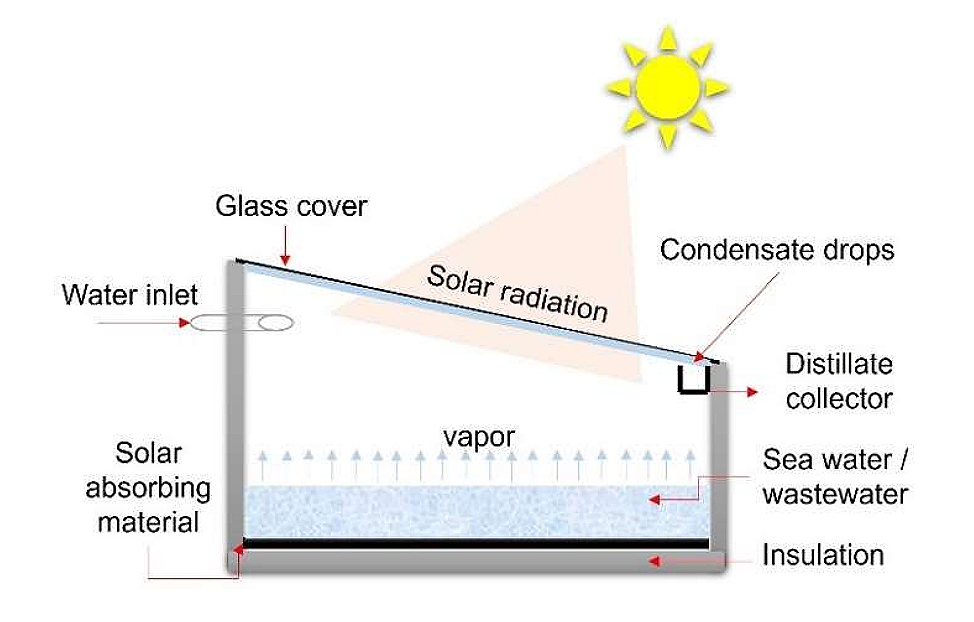 Figure 1. Schematic representation of a conventional solar desalination system. Adapted from [17].
Figure 1. Schematic representation of a conventional solar desalination system. Adapted from [17].
This paper presents an experimental investigation into a new design of a solar still (SS) with a concave glass cover. The objective is to reduce the system's internal volume and improve energy utilization to enhance the still's productivity and produce contaminant-free water for evaluation (specifically, water contaminated with Allura red dye). The study presents results obtained using two different construction materials for the solar still under the climatic conditions of Toluca, State of Mexico, Mexico.
The purpose of the experiment was to assess the effectiveness of the proposed solar still design. The evaluations took place in Toluca City, State of Mexico, Mexico, located at a latitude and longitude of 19.37337°N, 99.55822°W. A precision scale, specifically the OHAUS Scout Pro-Model SPX2201, was employed for the measurements.
The operating principle is based on that of a conventional solar still. The incoming solar radiation is transmitted through the surface of the glass cover and absorbed by the container, transforming it into heat and increasing the temperature of the water until it evaporates. The glass cover works as a condenser for water vapor, which, being concave, favors condensation from the periphery (circumference) to the center. With the glass funnel, the distillate is captured and recovered. This design aims to promote the capture of solar radiation, as well as take advantage of the heat generated in the system by reducing the internal air volume.
Two solar stills were designed and built based on the same design proposal. Kitchen pans were used for both, which were modified and adapted. The first one had a hemispherical shape with a concave condensation cover made of stainless steel (SSCC-SS), whereas the second one was designed with a cylindrical geometry and a concave condensation cover made of aluminum with Teflon coating (SSCC-AT), as shown in Figure 2. Table 1 presents the thermophysical properties of both materials. Based on these properties, it is expected that the behavior of each distiller will differ significantly, as aluminum has a greater capacity to conduct heat than stainless steel.
The SSCC-SS distiller is composed of a hemispherical base measuring 0.30 m in diameter and 0.09 m in height, designed to hold the contaminated water. In the center of this, a stainless-steel funnel of 0.12 m in diameter and 0.05 m in height with an inclination angle of 20° was fitted crossing the basin and is used to collect and exit the distillate. The condensation cover of the distiller is made of glass with a thickness of 6 mm, diameter of 0.30 m and inclination angle of 11°, placed concavely. On the other hand, the SSCC-AT solar still consists of a cylindrical metal container with a diameter of 0.34 m and a height of 0.075 m. In the center of the basin is a glass funnel with a diameter of 0.15 m, a height of 0.03 m and an angle of inclination of 10°. The condensation cover of the distiller is made of glass with a thickness of 6 mm, diameter of 0.36 m, and inclination angle of 14°. The cover is placed concavely. Both stills are sealed airtight with the use of a latex band that holds the base and cover together. The distillers are placed on a metal base, below which the container for recovering the distillate is connected. Both stills were placed on a homemade concentrator solar (CS), which consists of thick rectangular film (polystyrene with an aluminum outer film), which was placed around the still simulating a parabolic concentrator solar; a car sunshade reflective was used. Additionally, two DS18B20 sensors (Arduino Uno) were placed to measure the temperature of the water and internal air, which recorded the data per minute on a computer.
The volume of water in each distiller is 1 L. According to its dimensions, the SSCC-SS distiller has a total volume of 4.0 L while the SSCC-AT distiller has a volume of 4.7 L. Consequently, the inner air volume in each distiller is 3.0 L and 3.7 L respectively, accounting for 75% and 79% of the total volume for each distiller. In conventional stills such as single-slope or hemispherical stills, the inner air volume is ≈95% [32,33].
Experimental ProcedureThe experiment was carried out from June to September 2023. The daily productivity of the proposed solar still was obtained by experimenting from 08:00 a.m. to 06:00 p.m. To evaluate the efficiency in performance and removal of the Allura red dye (CAS: 25956-17-6), from synthetic water; a solution of 50 ppm of the dye was prepared using distilled water. Figure 3 shows photographs of both distillers in operation; condensation can be observed on the glass cover.
This was determined using the test method established in the NMX-AA-008-SCFI-2016 standard. The measuring instrument was Hanna Instruments HI98129, the measurements were carried out for initial synthetic water and the distillate obtained [34].
ConductivityThis was determined using the test method established in NMX-AA-093-SCFI-2018 standard. The measuring instrument was Hanna Instruments HI98129, whose measurement range is from 0 to 3999 µs/cm. The equipment was calibrated with a 1413 µs/cm standard [35].
Total organic carbon (TOC)This was determined using the test method established in NMX-AA-187-SCFI-2021 standard. The measuring instrument was TOC-L Shimadzu Total Organic Carbon analyzer, which uses the 680 °C combustion catalytic oxidation method, providing a range of 4 μg/L to 30,000 mg/L [36].
Total solidsThey were determined based on the NMX AA-034-SCFI-2015 standard. The determination was carried out in triplicate, first the porcelain capsule was placed in a Felisa oven model FE-291 AD for 20 min at 105 °C ± 2 °C, then it was cooled in a desiccator for 20 min and weighed in an analytical balance and the value was recorded as m1. Next, 50 mL of the sample to be evaluated was placed in the capsule and taken to the oven for complete evaporation at a temperature between 100 and 105 °C, cooled in a desiccator for 20 minutes, and finally the capsule was weighed in an analytical balance recording the value as m2. Calculations were made using the following formula:
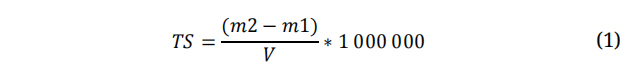
Where: TS = Total solids (mg/L)
m1 = mass of empty porcelain capsule at constant mass (g)
m2 = mass of the capsule with the residue after evaporation (g)
V = Sample volume (mL)
UV-VIS spectrophotometryTo determine the Allura red dye concentration a VELAB spectrophotometer was used, model VE-5100UV UV-VIS. Itis equipped with a silicon photodiode (detector), and the lamps are Deuterium (UV) and Tungsten (Visible). The measurements were executed across the spectrum ranging from 200 to 700 nm.
Figure 4 and Figure 5 show the variation in water temperature for both distillers with and without the concentrator solar (CS). It was noticed that both distillers took the same amount of time (between 1.5 to 2 h) to reach a temperature of 50 °C when not using them. From this point, an increase in the condensation rate was observed. In addition, the SSCC-AT remains above this temperature, reaching a maximum of 61 °C for a period between 4.4 and 5.5 hours, longer than the SSCC-SS, which remains between a period of 2.5 and 3.5 hours with a maximum of 55 °C. On the other hand, when using a concentrator solar in both SS, an increase in the average maximum temperature was observed for SSCC-AT, reaching 70 °C, while for SSCC-SS it was 68 °C. Additionally, both SS are kept above 50 °C for a period between 5 and 7 hours. The above shows that it is possible to maintain a more efficient continuous evaporation-condensation process when using the concentrator solar. Therefore, this was used for all tests since it manages to increase the system temperature by approximately 10 °C and keeps it stable for a longer period. The previously mentioned data coincide with works such as Attia, Kabeel et al. [37] with a hemispherical solar still with a steel tray and reflective mirrors, reached a water temperature of 74 °C compared to 68 °C for the reference still. Likewise, Attia et al. [33] in a recent study, using a hemispherical solar still, and a truncated cone-shaped reflecting mirrors, recorded the maximum water temperature of 76 °C with an inclination angle of 25° of the mirrors, which was higher than the reference distiller which recorded a maximum of 71 °C.
Regarding the temperature inside the still, Figure 6 shows the inner air and water temperatures with both stills. The temperature of the inner air is higher than that of the water by approximately 10 °C. When comparing these results with other designs using conventional geometry, a different behavior is observed, such as Attia M. et al. where in a hemispherical distiller the design and construction of reflective mirrors to achieve greater performance was carried out; the maximum inner air and water temperatures were 60° and 68 °C to 75 °C respectively [33]. Similarly, Pal P. and Dev R., in a conventional single-slope distiller with a modified base, recorded the inner air and water temperatures obtained, which were 60 °C and 70 °C respectively [32]. In both cases, there is a difference of approximately 10 °C, with the water temperature being higher than the air temperature. We relate this difference to the volume of inner air in the systems; in the distillers in this study the volume represents 75% to 78% of the total volume, and in conventional systems it ranges between 95% to 97%.
The efficiency of both distillers was evaluated at different initial volumes of synthetic water using equation (2). In the SSCC-SS/CS, the highest efficiency was obtained with an initial volume of 0.10 L, was 96%, and the lowest efficiency with an initial volume of 2 L was 10%. In the SSCC-AT/CS, the highest efficiency was obtained with an initial volume of 0.25 L, obtaining 89%, and the lowest efficiency with an initial volume of 2 L was 14%. Figure 7 shows the distillation efficiency at different volumes for both solar stills. It is observed that, by increasing the initial volume of synthetic water, the distillation efficiency decreases, this indicates that the heating rate of water in volumes greater than 0.25 L is not fast enough so that, during the radiation exposure, the evaporation-condensation process is not efficient. This limits the fact that these systems must work with a suitable layer of water according to the diameter of the solar still where the radiation is carried out. This limitation can be overcome using a continuous filing device to maintain a constant volume throughout the process.
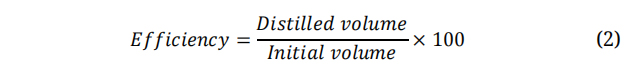
The quality of the distillate was evaluated by the elimination of the Allura red dye in the distillate, for this, the analytical technique of UV-Vis spectroscopy was used, in the distillates obtained with each distiller. Figure 8 and Figure 9 show the results obtained for the SSCC-AT/CS and SSCC-SS/CS solar still, respectively. These results are the average of 3 tests for each distiller. The initial concentration of Allura red dye is 50 mg/L. The presence of N-N azo bonds is observed at the wavelength of 505 nm [38].
According to Figure 8, the values obtained in the absorbance of the dye were less than 0.01 AU in the distillates from the SSCC-AT/CS distiller. This indicates that the presence of the dye is not observed in the distillate.
On the other hand, in Figure 9 the results of the solar still SSCC-SS/CS are presented. In this case the dye is in the distillate is in a concentration of 15 ± 5 mg/L. Therefore, the SSCC-AT/CS system provides a better recovered water quality.
This decrease in concentration indicates the presence of carryover of the dye in the distillate. To confirm this, laboratory tests were conducted by replacing solar heating with a heating plate while using a still system following the proposed operating principle. It was observed that once the water began to boil, the resulting condensation droplets showed the presence of dye. On the other hand, tests were conducted without the use of a solar concentrator, where maximum temperatures between 45 and 50 °C were recorded. No presence of colorant was observed in this distillate. Therefore, the use of the material "stainless steel" in this proposed design has a limitation for distilling the Allura red dye, because when the temperature increases rapidly it is dragged uncontrolled drops projection provoked by the fast evaporation of the water. In the case of the "aluminum with Teflon coating" distiller, it does not exhibit the behavior of dragging dye into the steam/drops, even though the records of maximum temperature reached with the SSCC-AT/CS range between 68 and 75 °C. This can be attributed to thermophysical properties such as thermal conductivity (Table 1), which value is much higher than that of stainless steel, resulting in more uniform heat transfer. Likewise, the heat capacity of aluminum is greater, requiring more energy to raise the temperature, making it more stable.
These results shows that it is necessary to explore the temperature where pollutants can be selectively separated in this solar still design. And uncontrolled process can promote undesired drag of polluting molecules. This is particularly important when pollutant molecules are colorless, making the distillate appear pure.
The quality of the distillate obtained with the SSCC-AT/SC was evaluated, because it was the one that showed the absence of color. It was compared with the initial synthetic water by measuring the previously mentioned parameters, as shown in Table 2.
It is observed that both the initial water and the distillate maintain the acid pH value of 6.72 and 6.51 respectively, which is within the permissible limit established by the NOM-127-SSA1-1994 standard (6.5 to 8.5) [39]. On the other hand, the electrolytic conductivity of the distillate decreased, indicating the removal of the dye that contributed to conductivity to the initial synthetic water.
Total organic carbon (TOC) showed a decrease, with a value of 0.53 mg/L for the distillate obtained. Finally, in the total solids it is observed that the distillate has a value of 6.3 mg/L, which is below the maximum permissible limit of 100 mg/L, as established by the NOM-067-ECOL-1994 standard [40].
Economic EvaluationThe cost of fabricating the SSCC-SS/CS and the SSCC-AT/CS (Table 3), was USD 85 and USD 80, respectively. The cost analysis was carried out using the method proposed by Kaviti A., et al. [25] to arrive at a cost per liter (CPL) in each case, assuming a lifetime of 5 years (y) and 250 sunny days (n). The mathematical formulas and parameters are listed in Table 4. The SSCC-SS/CS and SSCC-AT/CS had a greater daily distillate output (c) of 1.7 mL/m2/day and 3.29 mL/m2/day, and their CPLs were USD 0.06 and USD 0.03, respectively.
This study focused on developing a low-cost solar still, easy to build and operate. Therefore, the cylindrical geometry was proposed to take advantage of solar radiation from any solar orientation. In that sense, a concave cover was designed with distillate collection through the center of the basin. The concave cover not only modified the distillate collection system, it also decreased the volume of the solar still (inner air volume), compared to existing stills. The system was tested with synthetic water contaminated with Allura red dye.
The distillate water cost per liter is 0.06 and 0.03 USD for SSCC-AT/CS and SSCC-SS/CS respectively. This indicates that the proposed system is encouraging for real application.
Future work can be expanded with the recording of environmental parameters such as ambient temperature and solar radiation. Future research can be conducted by optimizing heat storage and transfer to improve efficiency. Additionally, develop and evaluate photocatalysts for the degradation of contaminants during distillation.
The dataset of the study is available from the authors upon reasonable request.
CBD, BFU conceptualization and project proposal. ARG final design, construction of the still systems and the experimental work. ARG, CBD, BFU and LAC contributed to the data interpretation and manuscript writing. All authors critically reviewed and approved the final version of the manuscript.
The authors declare that there is no conflict of interest.
The authors acknowledge the technical supports given by the Consejo Nacional de Humanidades Ciencias y Tecnologias (CONAHCYT, ARG CVU:700525), the Universidad Autónoma del Estado de México (UAEMex) and the Centro Conjunto de Investigación en Química Sustentable UAEM-UNAM to carry out this research. The authors recognized the technical support of Ing. Citlalit Martínez and Dra. Deysi Amado Piña.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
37.
38.
39.
40.
Ramos Garcia A, Barrera Diaz CE, Frontana Uribe BA, Ávila Córdoba LI. Low-Cost Solar Still System with Concave Condensing Cover for the Distillation of Synthetic Water Polluted with Allura Red Dye. J Sustain Res. 2024;6(2):e240036. https://doi.org/10.20900/jsr20240036

Copyright © 2024 Hapres Co., Ltd. Privacy Policy | Terms and Conditions