Location: Home >> Detail
TOTAL VIEWS
Regen Med Front. 2019;1:e190003. https://doi.org/10.20900/rmf20190003
1 Lady Davis Institute for Medical Research, Jewish General Hospital, Montréal, Québec H3T 1E2, Canada
2 Department of Human Genetics, McGill University, Montréal, Québec H3A 1B1, Canada
3 Department of Chemistry, McGill University, Montréal, Québec H3A 1B1, Canada
* Correspondence: Colin Crist, Tel.: +1-514-340-8222.
Background: Regeneration of adult tissues requires the activity of rare, mitotically quiescent somatic stem cells. These features are illustrated by the muscle stem cell (MuSC), also known as the satellite cell for its satellite position underneath the basal lamina of the myofiber. Isolation of MuSCs results in their rapid activation of the myogenic program and their subsequent culture ex vivo leads to loss of stem cell regenerative capacity. These shortcomings make MuSCs difficult to study, manipulate and prevent cell based therapies. We have previously shown that muscle stem cells (MuSCs) require tightly regulated protein synthesis through the phosphorylation of eIF2α. Sal003, an analog of salubrinal that blocks eIF2α dephosphorylation, promotes ex vivo expansion of MuSCs retaining regenerative capacity after engraftment into the Dmdmdx mouse model of Duchenne muscular dystrophy.
Methods: Since micromolar concentrations of sal003 (10 µM) are required to expand MuSCs ex vivo, we undertook a structure relations study to identify novel sal003 analogs with efficacy to increase phosphorylated eIF2α levels at lower concentrations. We demonstrate ex vivo expansion of MuSCs isolated from wild-type and mdx mice using new compounds.
Results: Here, we have synthesized and screened chemical analogs of sal003 to identify a novel compound promoting the ex vivo expansion of MuSCs. The novel compound expands wild-type and mdx MuSCs more efficiently than sal003 and also prolongs culture of primary myoblasts from isolated MuSCs.
Conclusions: We identify a novel sal003 analog, C10, with increased potency at lower concentrations. Culture conditions including sal003 or C10 can extend culture of primary myoblasts from isolated MuSCs, which we predict will enable their further study, genetic manipulation and cell based therapies.
MuSC, muscle stem cell; SAR, structure activity relations; ppm, parts per million; DMSO, dimethylsulfoxide; luc, luciferase; TA, tibialis anterior
Adult tissues with the capacity to regenerate do so by virtue of their somatic stem cells. The regeneration of skeletal muscle is facilitated by normally quiescent muscle stem cells (MuSCs), or “satellite cells”, named for their satellite position beneath the basal lamina of the myofibre [1], which are essential for post-natal repair of skeletal muscle [2–5]. Quiescent MuSCs normally express PAX7 and, in a subset of body muscle, PAX3 [6]. In response to injury, MuSCs activate the cell cycle and the expression of members of the myogenic regulatory family of transcription factors (MYF5, MYOD). MuSCs proliferate, differentiate to repair the injured muscle, and self-renew to re-establish the MuSC pool.
The niche plays an important role in maintaining MuSCs and isolation of MuSCs from their in vivo niche rapidly influences gene expression [7,8]. Furthermore, ex vivo culture of MuSCs results in their rapid entry into the myogenic program and loss of MuSC regenerative capacity [7]. Efforts to understand biophysical and molecular cues that regulate quiescence have been useful to mitigate the loss of MuSC regenerative capacity during ex vivo culture [8–11]. We have shown that the phosphorylation of eIF2α is a translational control mechanism regulating MuSC quiescence and self-renewal. Activated MuSCs dephosphorylate eIF2α, increase protein synthesis, and rapidly activate the myogenic program. Pharmacological inhibition of the eIF2α phosphatase Gadd34/PP1 by the small compound sal003 [12], a more potent derivative of salubrinal [13], permits the ex vivo expansion of MuSCs that retain regenerative capacity, as illustrated by engraftment into the Dmdmdx mouse model of Duchenne muscular dystrophy [10].
While salubrinal had been identified as a selective inhibitor of the eIF2α dephosphatase Gadd34/PP1 [13] (Figure 1A), the molecular mechanisms underlying its inhibition, and furthermore the mechanisms underlying the increased potency of sal003 [12] remain unclear. Previous structure activity relations (SAR) demonstrate that the tricholoromethyl groups of salubrinal are essential for activity, while the quinolone ring terminus is recognized as a key site for modification (R2 group) [14,15] Replacement of the R2 N-terminal quinolyl group of salubrinal with 4-chlorophenyl led to the more potent sal003 derivative [12].
Here, we ask whether the chemical structure of sal003 can be further modified to improve efficacy, concentrating on the 4-chlorophenyl group that replaced the quinolyl group of the parent salubrinal molecule. We identify a novel analogue of sal003 that expands MuSCs ex vivo at lower concentration. We also demonstrate culture conditions containing the novel sal003 analogue permit ex vivo expansion and passaging of primary adult MuSCs.
All chemicals and solvents were purchased from Sigma Aldrich (St. Louis, USA), Alfa Aesar (Haverhill, USA), TCI (Tokyo, Japan), or Oakwood Chemicals (Estill, USA). All solvents were dried and purified using an MBraun MB SPS 800 (Garching, Germany) or Innovative Technology PureSolv MD 7 (Amesbury, USA). Unless otherwise stated, reactions were performed in flame-dried glassware under a nitrogen or argon atmosphere. Column chromatography was conducted using 200–400 mesh silica gel from Silicycle (Quebec City, Canada). 1H-NMR spectra were acquired using Bruker Ascend 500 MHz, Bruker Ascend 400 MHz, and Varian Inova 400 MHz spectrometers. Chemical shifts (δ) are reported in parts per million (ppm) and are calibrated to the residual solvent peak. Coupling constants (J) are reported in Hz. Multiplicities are reported using the following abbreviations: s = singlet; d = doublet; t = triplet; q = quartet; m = multiplet (range of multiplet is given). 13C-NMR spectra were acquired using Bruker Ascend 125 MHz (Billerica, USA), Bruker Ascend 100 MHz, and Varian Inova 100 MHz spectrometers (Palo Alto, USA). Chemical shifts (δ) are reported in parts per million (ppm) and are calibrated to the residual solvent peak. Analytical thin-layer chromatography was performed on pre-coated 250 mm layer thickness silica gel 60 F254 plates (EMD Chemicals Inc., Burlington, USA). Details on the synthesis of sal003 derivatives is available in Supplementary Information.
MiceCare and handling of animals were in accordance with the federal Health of Animals Act, as practiced by McGill University and the Lady Davis Institute for Medical Research, McGill Animal Ethics and Compliance, Animal Use Protocol 2012-7026, May 2012. All mice are maintained on a C57BL/6 background. For bioluminescence assays, Tg(CAG-Luc-GFP) mice [16] (Jackson Laboratories, Bar Harbor, Maine, United States) were used. For engraftment assays, immunocompromised Foxn1nu/nu; Dmdmdx4cv/mdx4cv females and Foxn1nu/nu; Dmdmdx4cv/Y males (Jackson Laboratories) were used. MuSCs were isolated from abdominal and diaphragm muscle of 6- to 8-week old Pax3GFP/+ [17], Pax3GFP/+; Dmdmdx4cv mice using a FACSAriaIII cell sorter as previously described [10]. Alternatively, MuSCs were isolated from the abdominal and diaphragm muscle of 6- to 8-week old Tg(Cag-Luc-GFP) mice via the Miltenyi MACS Satellite Cell Isolation Kit (Bergisch Gladbach, Germany), following by positive selection with Miltenyi Anti-Integrin α7 beads. Isolated MuSCs were cultured in 39% DMEM, 39% F12, 20% FBS, 2% UltroserG, and when indicated, 0.1% DMSO (Control) and 5 µM C10, 10 µM sal003 or 100 nM thapsigargin, a potent inducer of endoplasmic reticulum stress and eIF2α phosphorylation. MuSC engraftment was performed as previously described [10]. Six- to eight-week-old Foxn1nu/nu; Dmdmdx mice were used as recipient mice for engraftment assays. Mice were exposed to 18Gy of irradiation to their right hindlimb muscle 24 h prior to receiving the engraftment. 10,000 cells were suspended in 10 µL PBS, loaded into a 10 µL Hamilton syringe (Reno, Nevada, United States) and introduced in a single injection longitudinally throughout the tibialis anterior (TA) muscle. Mice were euthanized 21 days after the engraftment and the TA muscle was isolated for immunodetection.
Cell Culture AssaysC2C12, HEK293 and HEK293T cells were cultured in DMEM with 10% FBS. The Atf4-luciferase construct was made by cloning the 5′ UTR of Atf4 upstream of the firefly luciferase gene in the pGL3 promoter plasmid, as described [18]. HEK293 cells were plated in 24-well plates at a density of 25,000 cells/well and incubated overnight. The Atf4-pGL3 plasmid and pRL-TK renilla luciferase plasmid were co-transfected into these cells using jetPRIME® transfection reagent (Illkirch-Graffenstaden, France) and incubated overnight. Compounds were reconstituted in DMSO and added to the cells for 16 h at 10 µM final concentration. Cells were then lysed and luciferase expression was measured using the Promega Dual-Luciferase Reporter kit (Madison, USA).
P-eIF2α levels were measured using the AlphaScreen® SureFire® eIF2α (P-Ser51) assay kit (Waltham, USA). C2C12 cells were added to 96-well plates at a density of 5000 cells/well, and cultured for 24 h. Compounds were added at a concentration of 10 µM for 4 h and then cells were lysed and lysates were transferred to a 384-well plate. Acceptor beads were incubated with cell lysates at room temperature for 1 h, and then donor beads were added and incubated overnight at room temperature in darkness. Emission signal was recorded using a PerkinElmer EnVision plate reader (Waltham, USA).
Luciferase Expansion Assay400 MuSCs from Tg(Cag-Luc,-GFP) mice were seeded on a 96-well gelatin-coated plate in MuSC medium with 10 µM of compounds, or DMSO (control). Bioluminescence (photons/second) was measured at the indicated intervals by replacing 10% of medium with MuSC medium supplemented with 1.5 mg/mL d-Luciferin (Gold Biotechnology, St. Louis, USA) and recorded using an IVIS Spectrum (Perkin Elmer, Waltham, USA).
ImmunodetectionImmunofluorescence labelling of cultured MuSCs and transverse sections of TA muscle was performed as previously described [10,19]. For immunoblotting, cell lysates were prepared as previously described [20]. ImageJ was used to determine the densitometry from immunoblots. Primary mouse antibodies against PAX7 (DSHB), MYH1E (DSHB, MF20, Iowa City, USA), MYOGENIN (Abcam, EPR4789, Cambridge, UK), β-ACTIN (Sigma, AC-15, St. Louis, USA), P-eIF2α (Abcam, E90), total eIF2α (Cell Signaling, L57A5, Danvers, USA) and rabbit antibodies MYOD (Abcam), DYSTROPHIN (Pierce, Waltham, USA), GFP (Life Technologies, Carlsbad, USA). Secondary antibodies are Alexa Fluor 488 and 594 anti-mouse antibodies (Thermo Fisher Scientific, Waltham, USA), Alexa Fluor 488 and 594 anti-rabbit antibodies, and Horseradish peroxidase (HRP) conjugated goat anti-mouse or anti-rabbit antibodies (Jackson Immunoresearch, West Grove, USA). For immunoblotting, ECL Prime Western Blotting Detection reagents (GE Healthcare, Marlborough, USA) and detected with an ImageQuant LAS 4000 (GE Healthcare, Chicago, Illinois, United States).
RNA AnalysisRNA was isolated from cells in culture using TRIzol reagent (Life Technologies) and treated with DNase (Roche, Basel, Switzerland). RNA was reverse transcribed using the Superscript III reverse transcriptase (Life Technologies) using oligoDT primers. Primers are Pax7 Forward (FWD) 5′-CTCAGTGAGTTCGATTAGCCG-3′, Reverse (REV) 5′-AGACGGTTCCCTTTGTCGC-3′; MyoD FWD 5′-CCCCGGCGGCAGAATGGCTACG-3′, REV 5′-GGTCTGGGTTCCCTGTTCTGTGT-3′; MyoG FWD 5′-CAACCAGGAGGAGCGCGATCTCCG-3′, REV 5′-AGGCGCTGTGGGAGTTGCATTCACT-3′; and Actb FWD 5′-AAACATCCCCCAAAGTTCTAC-3′, REV 5′-AAACATCCCCCAAAGTTCTAC-3′.
Passaging MuSCsMuSCs were isolated by FACS as previously described and plated on 35 mm gelatin-coated dishes at a density of 7500 cells/plate. After 3 days in culture, cells were washed with PBS once and trypsinized with 0.05% trypsin at 37 °C for 1 min. Cells were resuspended in 1 mL 10% FBS, F12 medium and centrifuged at 600× g for 10 min at 4 °C. Pellets were resuspended in 100 µL MuSC medium, counted with a haemocytometer and plated at 2000 cells/plate for wild-type and 1000 cells/plate for Dmdmdx. Cells were subsequently passaged every 2 days, following the same protocol.
Statistical AnalysisGraphical analysis is presented as mean ± SEM. At least three independent replicates of each experiment were performed. Unless otherwise indicated, significance was calculated using unpaired Student’s t-tests with two-tailed p values: * p < 0.05, ** p < 0.01, *** p < 0.001.
To identify more efficacious analogues of sal003 (Figure 1A), we synthesized a library of derivatives (Figure 1B). Using a modified route previously described [15], a panel of analogues bearing electronically and sterically diverse substituents at R2, which corresponds to the region of sal003 that was modified to improve efficacy, were synthesized. Aside from C8, all analogues were comprised of the trichloromethyl (-CCl3) core as this functional group had previously been shown to be crucial for activity [14,15]. With this in mind, a variety of thiourea-based aryl groups were incorporated at R2 containing both electron-donating and withdrawing groups at differing positions.
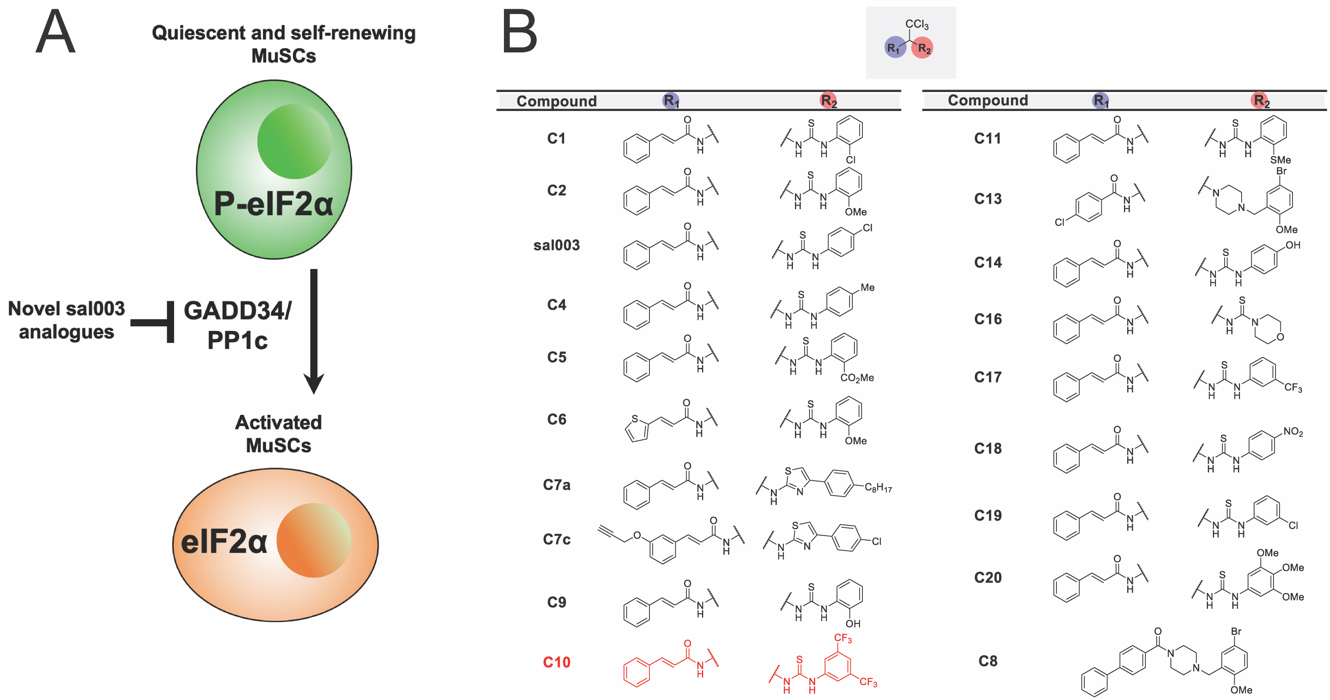 Figure 1. Identification of more efficacious analogues of sal003. (A) A schematic illustrating the study objective to identify novel analogues of sal003 that inhibit eIF2α dephosphorylation to increase MuSC self-renewal during ex vivo culture. (B) Library of sal003 derivatives. The lead compound, C10, is highlighted in red.
Figure 1. Identification of more efficacious analogues of sal003. (A) A schematic illustrating the study objective to identify novel analogues of sal003 that inhibit eIF2α dephosphorylation to increase MuSC self-renewal during ex vivo culture. (B) Library of sal003 derivatives. The lead compound, C10, is highlighted in red.
To screen our panel of novel compounds for activity, we constructed a plasmid vector containing the 5′ UTR of Atf4, which contain two open reading frames that control Atf4 mRNA translation in a P-eIF2α dependent manner [18], fused to a firefly luciferase reporter (Figure 2A). 293 cells were transiently transfected with the Atf4-fluc reporter, and treated with sal003 and our panel of novel compounds. We identified four hits that increased P-eIF2α dependent translation of the Atf4-fluc reporter above levels achieved by sal003, of which compound 10 (C10, Figure 1B, red) is identified as our lead compound (Figure 2B). C10 has a 3,5-bis(trifluoromethyl) substituted for the p-chloro on the sal003 N-phenyl group at R2 (Figure 1). Next, we used a quantitative Alpha assay in C2C12 myoblasts to directly determine eIF2α levels after exposure to sal003, C10, a subset of compounds that did not increase translation of Atf4-luc reporter (C4, C5, C9 and C11) and 100 nM thapsigargin (an ER stress inducer that elevates P-eIF2α). Exposure to C10 resulted in significantly increased levels of P-eIF2α compared to sal003 (Figure 2C), and these results were confirmed by quantitative western blotting of the P-eIF2α to total eIF2α ratio (Figure 2D, see also Supplementary Figure S1A).
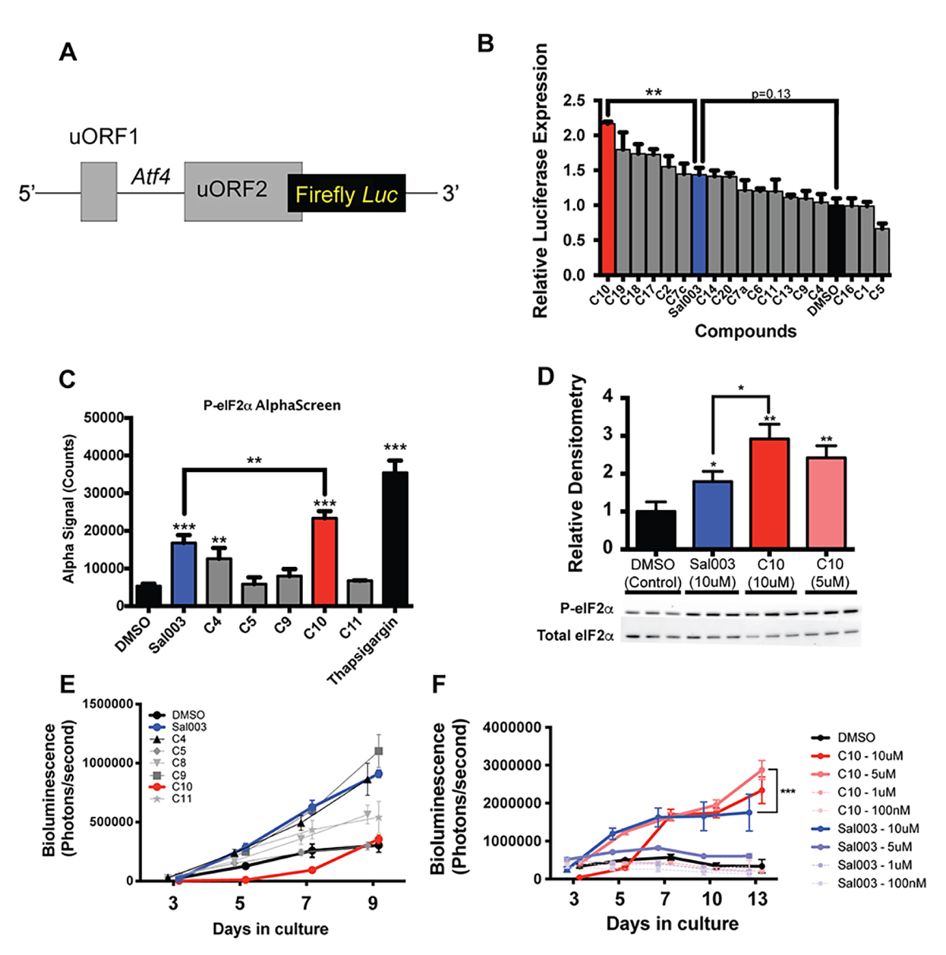 Figure 2. Identification of C10 as a candidate novel small compound inhibitor of eIF2α dephosphorylation. (A) Schematic illustration of the Atf4-luc reporter used to screen sal003 derivatives. (B) Relative Firefly:Renilla luciferase activity in 293 cells transfected with pGL3-Atf4-luc (firefly) and pRL-TK (Renilla) after 16 h culture with the indicated compounds at 10 µM. (C) P-eIF2α levels, determined by AlphaScreen SureFire eIF2α (Ser-51) assay, in C2C12 myoblasts after 4 h culture with 10 μM candidate compounds or DMSO (control). (D) Western blotting against P-eIF2α from cell lysates of C2C12 cells cultured for 4 h in C10, Sal003 or DMSO (control). (E) Bioluminescence of MuSCs isolated from Tg(Cag-luc-GFP) adult mice in the presence of 10 µM of the indicated sal003 analogs. (F) Bioluminescence from MuSCs isolated from Tg(Cag-luc-GFP) adult mice cultured with C10 and sal003 at the indicated concentration, or with DMSO (control). Values for Figure 2B,D indicate mean (n = 3) ± SEM, and values for Figure 2C,E,F indicate mean (n = 4) ± SEM. Significance was calculated using a One-way Anova with Bonferroni correction (2B,C), multiple two-tailed Student’s t-tests (2D), and two-way Anova (Tukey) (2F). * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 2. Identification of C10 as a candidate novel small compound inhibitor of eIF2α dephosphorylation. (A) Schematic illustration of the Atf4-luc reporter used to screen sal003 derivatives. (B) Relative Firefly:Renilla luciferase activity in 293 cells transfected with pGL3-Atf4-luc (firefly) and pRL-TK (Renilla) after 16 h culture with the indicated compounds at 10 µM. (C) P-eIF2α levels, determined by AlphaScreen SureFire eIF2α (Ser-51) assay, in C2C12 myoblasts after 4 h culture with 10 μM candidate compounds or DMSO (control). (D) Western blotting against P-eIF2α from cell lysates of C2C12 cells cultured for 4 h in C10, Sal003 or DMSO (control). (E) Bioluminescence of MuSCs isolated from Tg(Cag-luc-GFP) adult mice in the presence of 10 µM of the indicated sal003 analogs. (F) Bioluminescence from MuSCs isolated from Tg(Cag-luc-GFP) adult mice cultured with C10 and sal003 at the indicated concentration, or with DMSO (control). Values for Figure 2B,D indicate mean (n = 3) ± SEM, and values for Figure 2C,E,F indicate mean (n = 4) ± SEM. Significance was calculated using a One-way Anova with Bonferroni correction (2B,C), multiple two-tailed Student’s t-tests (2D), and two-way Anova (Tukey) (2F). * p < 0.05, ** p < 0.01, *** p < 0.001.
Next, we monitored the ability of novel compounds to expand ex vivo MuSCs isolated from skeletal muscle of adult Tg(Cag-luc-GFP) mice, where the expansion of colonies can be observed by bioluminescence (Figure 2E). Interestingly, one novel compound, C9, which has an o-hydroxyl substituted for the p-chloro on the sal003 N-phenyl group at R2 (Figure 1), caused a rapid increase in bioluminescence (Figure 2E), without increasing P-eIF2α dependent Atf4-luc translation (Figure 2B) or P-eIF2α levels (Figure 2C). Meanwhile the compound C10 resulted in poor MuSC expansion at 10 µM. We reasoned that excessively high P-eIF2α levels in the presence of 10 µM C10 would account for an overall decrease in bioluminescence by promoting lower rates of proliferation, as we have previously shown for sal003 [10]. We therefore titrated C10 and sal003 from 10 µM to 100 nM to show that 5 µM C10 potently expands MuSCs (Figure 2F) at P-eIF2α levels that are more equivalent to 10 µM sal003 (Figure 2D). In contrast, 5 µM sal003 does not expand MuSCs ex vivo (Figure 2F).
Inhibition of eIF2α Dephosphorylation by the Novel sal003 Analog C10 Promotes MuSC Self-Renewal during ex vivo CultureWe next examined whether 5 µM C10 delays the activation of the myogenic program during ex vivo culture. We isolated MuSCs from abdominal and diaphragm muscle of adult Pax3GFP/+ mice and cultured them for four days under normal conditions, or in the presence of 5 µM C10, and subsequently immunolabelled with antibodies against PAX7, MYOD, and MYOGENIN. Culture in the presence of 5 µM C10 over four days resulted in a 2-fold increase in the numbers of PAX7(+); MYOD(−) cells that have not activated the myogenic program, a 1.3-fold increase in PAX7(+); MYOD(+) cells that have activated the myogenic program, and a 1.5-fold decrease in numbers of differentiating PAX7(−), MYOD(+) cells (Figure 3A,B). Decreased differentiation of MuSCs cultured in the presence of 5 µM C10 was also reflected by a decrease in MYOGENIN(+) cells (Figure 3C,D). Immunoblotting with antibodies against PAX7 and MYOGENIN revealed a 3-fold increase in PAX7 and a 4-fold decrease in MYOGENIN levels after 4 days in culture with 5 µM C10 (Figure 3E,F, see also Supplementary Figure S1B,C). In the presence of C10, MyoD and Myogenin mRNA levels were also reduced. Conversely, Pax7 mRNA levels did not change (Figure 3G) to reflect the observed increase in PAX7 protein levels, which is similar to our previous observations with sal003 [10].
We next asked whether MuSCs expanded ex vivo in the presence of C10 retain their properties to self-renew and regenerate muscle after engraftment into the Dmdmdx mouse model of Duchenne muscular dystrophy. We isolated MuSCs from muscle of adult Pax3GFP/+ mice and cultured for 4 days in the presence of 5 µM C10. After this period of ex vivo culture, cells were engrafted into 18Gy irradiated hindlimbs of Dmdmdx/mdx; Foxn1nu/nu immunodeficient mice. Engraftment of either 10,000 C10-treated MuSCs, or newly isolated MuSCs, led to higher numbers of DYSTROPHIN(+) myofibres (Figure 4A,B) and PAX7(+), GFP(+) cells of donor origin (Figure 4C,D), when compared to 4-day culture of MuSCs in the presence of DMSO.
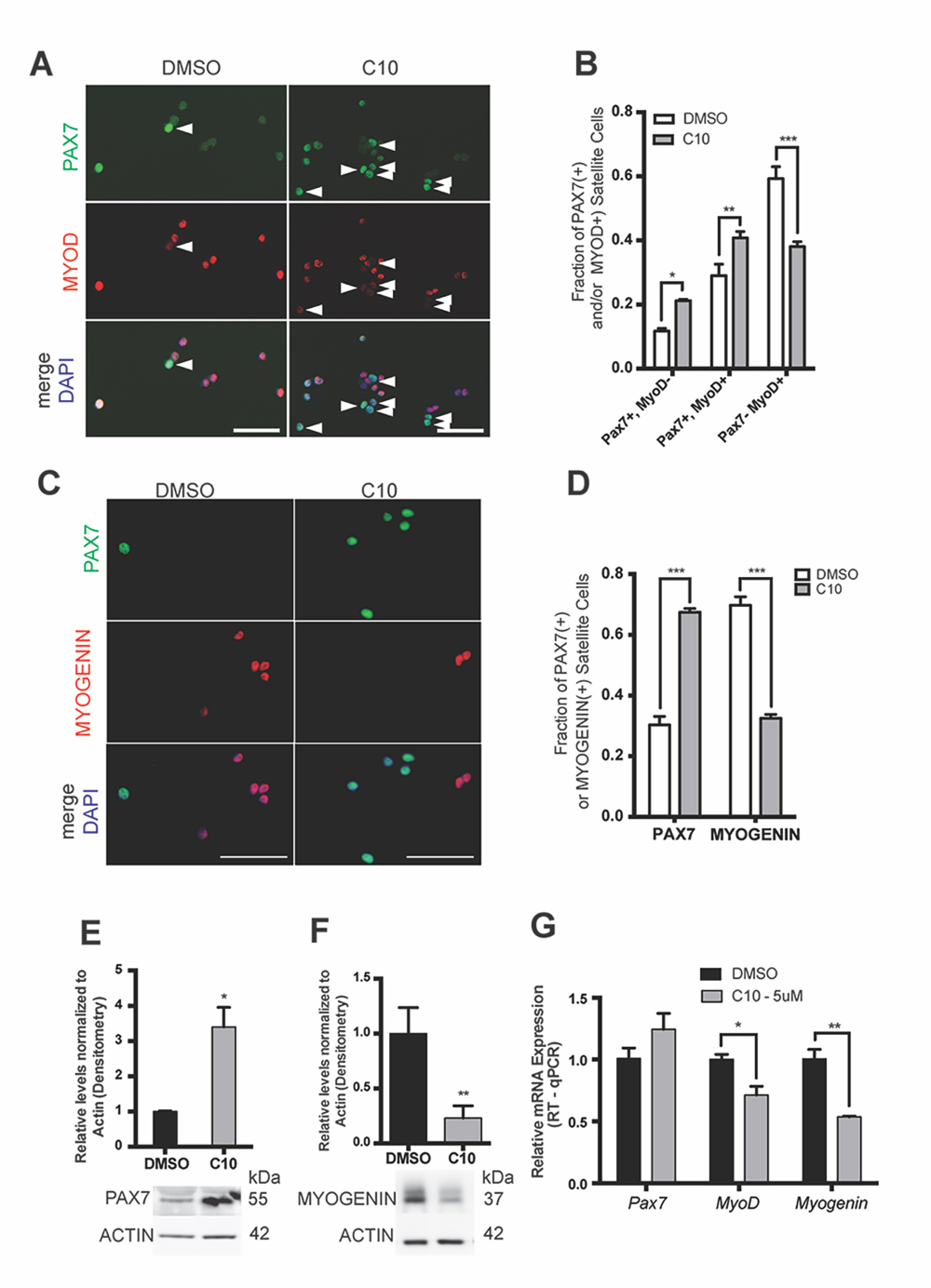 Figure 3. MuSCs cultured in C10 have delayed differentiation and improved self-renewal ex vivo. (A) Immunofluorescence with antibodies against PAX7 (Green) and MYOD (Red) on MuSCs isolated from Pax3GFP/+ mice after 4 day culture in 5 μM C10 or DMSO (control). Arrowheads to the right of nuclei (white) indicate presence of PAX7-positive, MYOD-negative cells. (B) Numbers of PAX7-expressing and MYOD-expressing cells in (A). (C) Immunofluorescence with antibodies against PAX7 (Green) and MYOGENIN (Red) on MuSCs isolated from Pax3GFP/+ mice after 4 day culture in C10 or DMSO (control). (D) Numbers of PAX7-expressing and MYOGENIN-expressing cells in (C). Scale bars in (A) and (C) represent 50 μM. (E,F) Western blotting with antibodies against PAX7, MYOGENIN and β-ACTIN of MuSC lysates after 4 day culture in C10 or DMSO (control). Levels are reported from densitometry of n = 3 western blots, normalized to ACTIN and relative to DMSO (control) cultures. (G) Relative mRNA levels of Pax7, MyoD and Myogenin by RT-qPCR after 4 day culture of MuSCs in C10 or DMSO (control). Pax7, MyoD and Myogenin mRNA levels are normalized to actb and are relative to DMSO (control) cultures. All values indicate mean (n = 3) ± SEM, significance was calculated using two-tailed Student’s t-tests, * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 3. MuSCs cultured in C10 have delayed differentiation and improved self-renewal ex vivo. (A) Immunofluorescence with antibodies against PAX7 (Green) and MYOD (Red) on MuSCs isolated from Pax3GFP/+ mice after 4 day culture in 5 μM C10 or DMSO (control). Arrowheads to the right of nuclei (white) indicate presence of PAX7-positive, MYOD-negative cells. (B) Numbers of PAX7-expressing and MYOD-expressing cells in (A). (C) Immunofluorescence with antibodies against PAX7 (Green) and MYOGENIN (Red) on MuSCs isolated from Pax3GFP/+ mice after 4 day culture in C10 or DMSO (control). (D) Numbers of PAX7-expressing and MYOGENIN-expressing cells in (C). Scale bars in (A) and (C) represent 50 μM. (E,F) Western blotting with antibodies against PAX7, MYOGENIN and β-ACTIN of MuSC lysates after 4 day culture in C10 or DMSO (control). Levels are reported from densitometry of n = 3 western blots, normalized to ACTIN and relative to DMSO (control) cultures. (G) Relative mRNA levels of Pax7, MyoD and Myogenin by RT-qPCR after 4 day culture of MuSCs in C10 or DMSO (control). Pax7, MyoD and Myogenin mRNA levels are normalized to actb and are relative to DMSO (control) cultures. All values indicate mean (n = 3) ± SEM, significance was calculated using two-tailed Student’s t-tests, * p < 0.05, ** p < 0.01, *** p < 0.001.
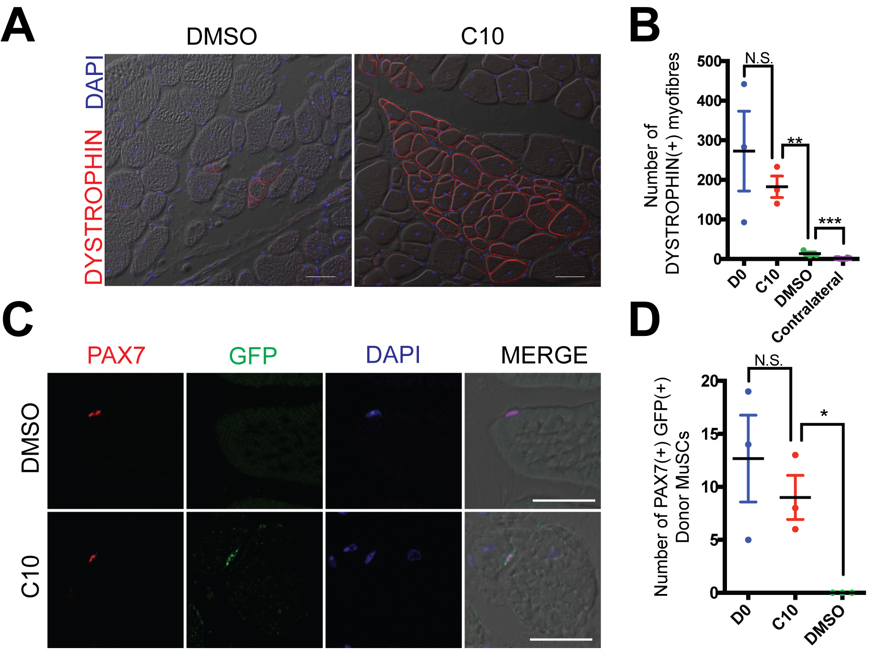 Figure 4. MuSCs maintain their engraftment capacity after 4-day culture in C10. (A) DYSTROPHIN (Red) immunolabelling of TA muscle 21 days after engraftment of 10,000 MuSCs cultured for four days with DMSO (control) or 5 μM C10. Nuclei are counterstained in DAPI (Blue). Scale Bars represents 50 μM. (B) Number of DYSTROPHIN(+) muscle fibres per immunolabelled cross-section from (A). Four day cultured cells are compared with engraftment of 10,000 fresh isolated MuSCs (D0). (C) Immunolabeling with antibodies against PAX7 (Red) and GFP (Green) on transverse sections of TA muscle following intramuscular engraftment of MuSCs cultured in DMSO (control) or C10 5 μM. Nuclei are counterstained in DAPI (Blue) Scale bars represent 25 μM. (D) Numbers of PAX7(+) GFP(+) donor derived cells per cross-section from (C). Four day cultured cells are compared with engraftment of 10,000 fresh isolated MuSCs (D0). All values indicate mean (n = 3) ± SEM, significance was calculated using multiple two-tailed Student’s t-tests, * p < 0.05,
** p < 0.01, *** p < 0.001, n.s. not significant.
Figure 4. MuSCs maintain their engraftment capacity after 4-day culture in C10. (A) DYSTROPHIN (Red) immunolabelling of TA muscle 21 days after engraftment of 10,000 MuSCs cultured for four days with DMSO (control) or 5 μM C10. Nuclei are counterstained in DAPI (Blue). Scale Bars represents 50 μM. (B) Number of DYSTROPHIN(+) muscle fibres per immunolabelled cross-section from (A). Four day cultured cells are compared with engraftment of 10,000 fresh isolated MuSCs (D0). (C) Immunolabeling with antibodies against PAX7 (Red) and GFP (Green) on transverse sections of TA muscle following intramuscular engraftment of MuSCs cultured in DMSO (control) or C10 5 μM. Nuclei are counterstained in DAPI (Blue) Scale bars represent 25 μM. (D) Numbers of PAX7(+) GFP(+) donor derived cells per cross-section from (C). Four day cultured cells are compared with engraftment of 10,000 fresh isolated MuSCs (D0). All values indicate mean (n = 3) ± SEM, significance was calculated using multiple two-tailed Student’s t-tests, * p < 0.05,
** p < 0.01, *** p < 0.001, n.s. not significant.
Primary myoblasts have limited replicative potential, are prone to differentiation, and eventually become senescent in culture. We therefore wanted to determine whether culture conditions including 5 μM C10 can extend the utility of primary myoblasts from isolated MuSCs. Isolated MuSCs were seeded on 35 mm culture dishes (7500 cells/plate) and cultured in the presence of 5 µM C10 or under normal conditions. Every three days, cells were passaged at a density of 1000 cells per 35 mm plate. 7500 MuSCs cultured in the presence of 5 µM C10 or 10 µM sal003 yielded greater than 3 million cells after three passages, compared to an average of 230,000 cells remaining after a maximum of two passages under normal culture conditions (Figure 5A). After 2 passages, the fraction of PAX7(+) cells is increased by three-fold in the presence of 5 µM C10, while the number of MYOGENIN(+) cells is decreased (Figure 5B,C). After one single passage, extended culture of primary myoblasts in the presence of C10 leads to their eventual differentiation and fusion into myotubes that are larger in diameter (Figure 5D) and exhibit a higher fusion index (number of DAPI counterstained nuclei per myotube) than normal culture conditions (Figure 5D,E). Even under these extended culture conditions, the number of PAX7(+) cells that are associated with myotubes are increased 3-fold in the presence of C10 (Figure 5D,F).
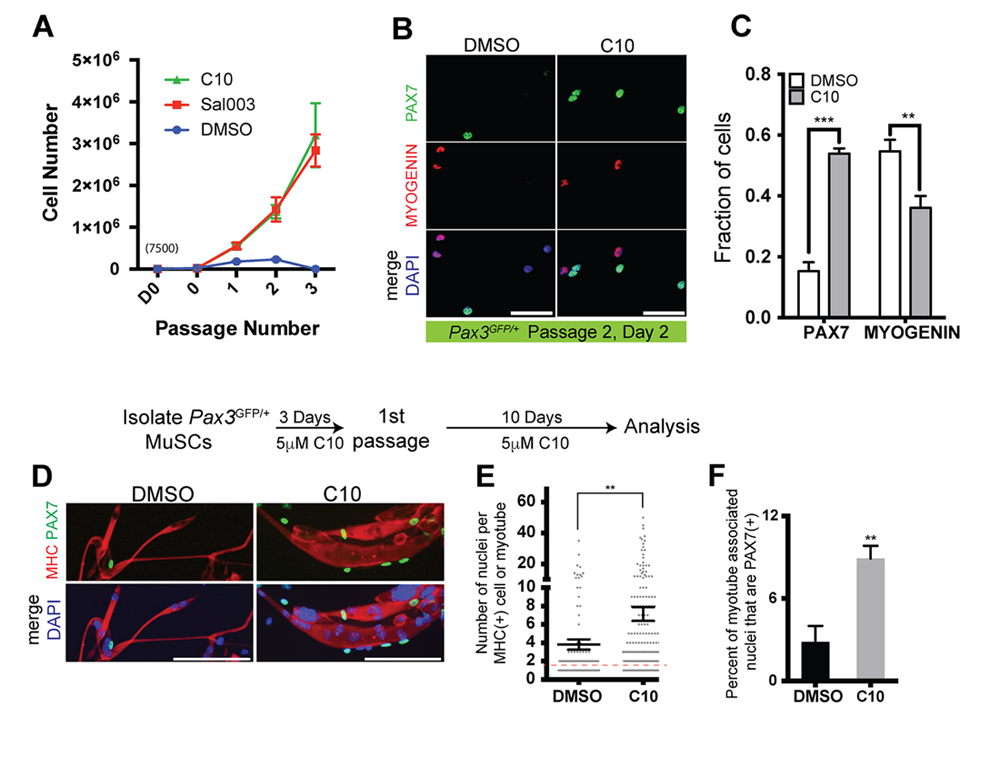 Figure 5. Expansion and passaging of MuSCs in C10. (A) Expansion of MuSCs derived from wild-type mice. MuSCs isolated from adult Pax3GFP/+ mice are plated at an initial density of 7500 cells/plate on day 0 (D0, 7500 cells; 0, number of cells after 3 days culture and prior to first passage), passaged at a density of 2000 after 3 days, and then passaged every 3 days at a density of 2000 cells/plate for subsequent passages. (B) Immunolabelling for PAX7 (Green) and MYOGENIN (Red) of wild-type MuSCs after passage 2, cultured with DMSO (control) or 5 µM C10. Scale bars represent 50 μM unless indicated otherwise. (C) Quantification PAX7(+) or MYOGENIN(+) MuSCs cultured in DMSO (control) or C10 from (B). (D) Immunolabelling for PAX7 (Green), Myosin Heavy Chain (MHC; Red) of cells cultured in the presence of C10 for 10 days following passage 1, permitting spontaneous differentiation. Scale Bars represent 100 μM. (E) Quantification of the number of DAPI+ nuclei per MHC+ myotubes from (D), values indicate mean of n = 98 cells/myotubes, DMSO; n = 188 cells/myotubes, C10 ± SEM. Red dotted line, unfused MHC(+) myoblasts. (F) Quantification of PAX7(+) nuclei associated with MHC myotubes from (D). All values, except in (E) indicate mean (n = 3 plates) ± SEM, significance was calculated using two-tailed Student’s t-tests. ** p < 0.01, *** p < 0.001.
Figure 5. Expansion and passaging of MuSCs in C10. (A) Expansion of MuSCs derived from wild-type mice. MuSCs isolated from adult Pax3GFP/+ mice are plated at an initial density of 7500 cells/plate on day 0 (D0, 7500 cells; 0, number of cells after 3 days culture and prior to first passage), passaged at a density of 2000 after 3 days, and then passaged every 3 days at a density of 2000 cells/plate for subsequent passages. (B) Immunolabelling for PAX7 (Green) and MYOGENIN (Red) of wild-type MuSCs after passage 2, cultured with DMSO (control) or 5 µM C10. Scale bars represent 50 μM unless indicated otherwise. (C) Quantification PAX7(+) or MYOGENIN(+) MuSCs cultured in DMSO (control) or C10 from (B). (D) Immunolabelling for PAX7 (Green), Myosin Heavy Chain (MHC; Red) of cells cultured in the presence of C10 for 10 days following passage 1, permitting spontaneous differentiation. Scale Bars represent 100 μM. (E) Quantification of the number of DAPI+ nuclei per MHC+ myotubes from (D), values indicate mean of n = 98 cells/myotubes, DMSO; n = 188 cells/myotubes, C10 ± SEM. Red dotted line, unfused MHC(+) myoblasts. (F) Quantification of PAX7(+) nuclei associated with MHC myotubes from (D). All values, except in (E) indicate mean (n = 3 plates) ± SEM, significance was calculated using two-tailed Student’s t-tests. ** p < 0.01, *** p < 0.001.
We asked whether MuSCs isolated from the Dmdmdx mouse model of Duchenne muscular dystrophy could be similarly expanded in the presence of C10. We isolated MuSCs from abdominal and diaphragm muscle of adult Pax3GFP/+; Dmdmdx (mdx) mice and cultured in the presence of 5 µM C10 or under normal conditions. Four day culture of mdx MuSCs in the presence of C10 yields a two fold increase in numbers of PAX7(+) cells, a two-fold decrease in MYOGENIN(+) nuclei (Figure 6A,B) and a two fold increase in numbers of PAX7(+); MYOD(−) cells that have not activated the myogenic program (Figure 6C,D).
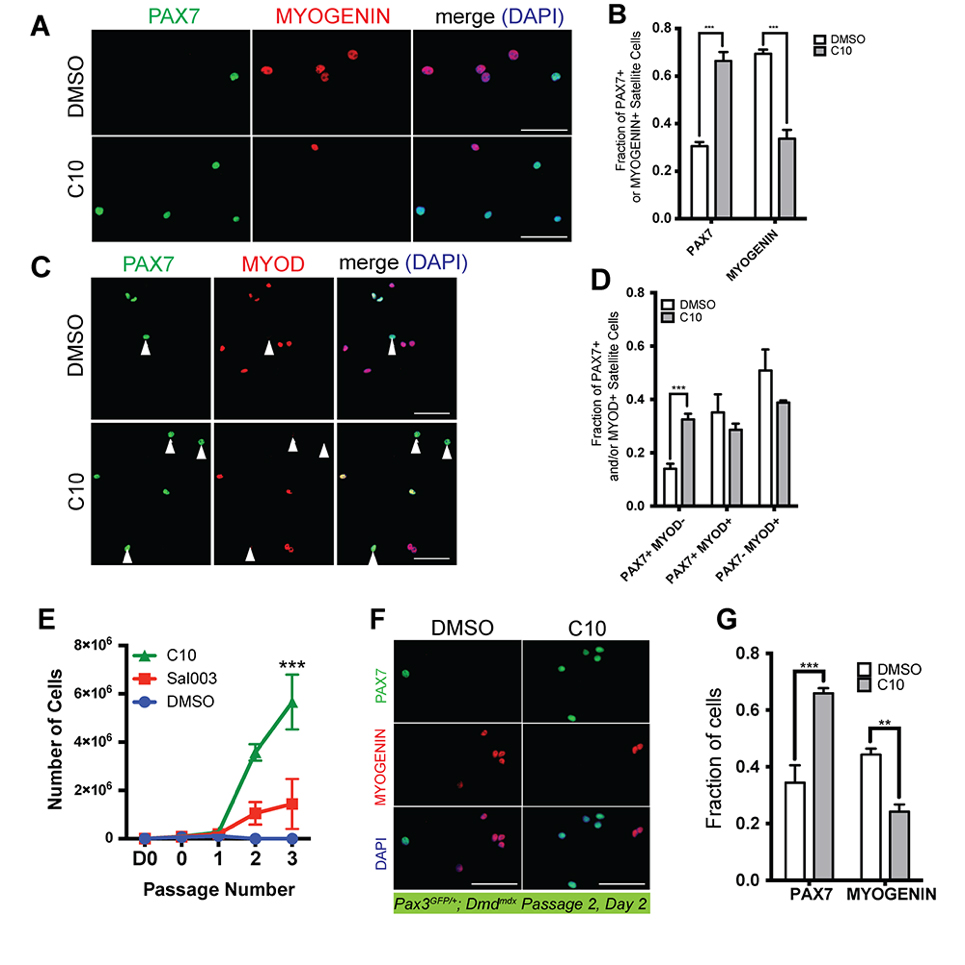 Figure 6. Ex vivo expansion and passaging of MuSCs isolated from Pax3GFP/+; Dmdmdx mice and cultured in the presence of C10. (A) Immunofluorescence with antibodies against PAX7 (Green) and MYOGENIN (Red) on MuSCs isolated from Pax3GFP/+; Dmdmdx mice after 4 day culture with 5 μM C10 or DMSO (control). (B) Quantification PAX7(+) or MYOGENIN(+) MuSCs cultured in DMSO (control) or C10 from (A). (C) Immunofluorescence with antibodies against PAX7 (Green) and MYOD (Red) on MuSCs isolated from Pax3GFP/+; Dmdmdx mice after 4 day culture with C10 or DMSO (control). (D) Quantification PAX7- or MYOD-expressing MuSCs cultured in DMSO (control) or C10 from (C). Scale bars represents 50 μM. (E) Expansion of MuSCs isolated from muscle of adult Dmdmdx mice for three passages. MuSCs isolated from adult Pax3GFP/+; Dmdmdx mice are plated at an initial density of 7500 cells/plate on day 0 (D0, 7500 cells; 0, number of cells after 2 days culture and prior to first passage), then passaged at a density of 2000 cells/plate every 2 days. (F) Immunolabelling for PAX7 (Green) and MYOGENIN (Red) of Pax3GFP/+; Dmdmdx MuSCs after two passages in the presence of DMSO (control) or 5 µM C10. (G) Quantification of PAX7(+) or MYOGENIN(+) MuSCs cultured in DMSO (control) or C10 from (F). All values indicate mean (n = 3) ± SEM. Significance was calculated using two-tailed Student’s t-test (B, D and G), and a two-way Anova (Tukey) (E), ** p < 0.01, *** p < 0.001.
Figure 6. Ex vivo expansion and passaging of MuSCs isolated from Pax3GFP/+; Dmdmdx mice and cultured in the presence of C10. (A) Immunofluorescence with antibodies against PAX7 (Green) and MYOGENIN (Red) on MuSCs isolated from Pax3GFP/+; Dmdmdx mice after 4 day culture with 5 μM C10 or DMSO (control). (B) Quantification PAX7(+) or MYOGENIN(+) MuSCs cultured in DMSO (control) or C10 from (A). (C) Immunofluorescence with antibodies against PAX7 (Green) and MYOD (Red) on MuSCs isolated from Pax3GFP/+; Dmdmdx mice after 4 day culture with C10 or DMSO (control). (D) Quantification PAX7- or MYOD-expressing MuSCs cultured in DMSO (control) or C10 from (C). Scale bars represents 50 μM. (E) Expansion of MuSCs isolated from muscle of adult Dmdmdx mice for three passages. MuSCs isolated from adult Pax3GFP/+; Dmdmdx mice are plated at an initial density of 7500 cells/plate on day 0 (D0, 7500 cells; 0, number of cells after 2 days culture and prior to first passage), then passaged at a density of 2000 cells/plate every 2 days. (F) Immunolabelling for PAX7 (Green) and MYOGENIN (Red) of Pax3GFP/+; Dmdmdx MuSCs after two passages in the presence of DMSO (control) or 5 µM C10. (G) Quantification of PAX7(+) or MYOGENIN(+) MuSCs cultured in DMSO (control) or C10 from (F). All values indicate mean (n = 3) ± SEM. Significance was calculated using two-tailed Student’s t-test (B, D and G), and a two-way Anova (Tukey) (E), ** p < 0.01, *** p < 0.001.
Next, MuSCs were isolated from muscle of adult Pax3GFP/+; Dmdmdx mice and seeded on 35 mm culture dishes (7500 cells/plate). Cells were cultured in the presence of 5 µM C10 or under normal conditions and passaged at a density of 2000 cells per 35 mm plate every two days. 7500 mdx MuSCs maintained in the presence of 5 µM C10 yielded on average 5.6 million cells after 3 passages, compared to an average yield of 1.4 million cells in the presence of 10 µM sal003 and only 100,000 cells after a maximum of two passages under normal culture conditions (Figure 6E). After 2 passages, the fraction of PAX7(+) mdx cells is increased in the presence of C10, while the number of MYOGENIN(+) mdx cells is decreased (Figure 6F,G).
Culture of MuSCs ex vivo leads to their rapid loss of regenerative capacity, as illustrated by their loss of engraftment capacity [7,8]. The inability to expand MuSCs that maintain regenerative capacity during ex vivo culture also places limitations on the study of underlying mechanisms of MuSC self-renewal, and limits their utility in bioengineering and cell based therapies. We and others have identified small compounds that enable ex vivo expansion of MuSCs [10,11]. In this work, we further our investigation into sal003 [10] with the goal to identify novel sal003 analogs with higher efficacy at lower concentrations.
Primary Screen for sal003 DerivativesUsing an Atf4-luc assay as a reporter of P-eIF2α dependent translation, as well as western blotting and Alpha-Screen to directly assess levels of P-eIF2α, we have screened a panel of analogues bearing electronically and sterically diverse substituents at R2, which corresponds to the region of sal003 that was modified to improve efficacy. These assays revealed the novel compound C10, which has a 3,5-bis(trifluoromethyl) substituted for the p-chloro on the sal003 N-phenyl group at R2 (Figure 1), is more potent to maintain P-eIF2α.
Potential Applications of C10 Culture Conditions for ex vivo Expansion of MuSCsExpansion of MuSCs ex vivo with 10 µM sal003 initially results in lower rates of proliferation, but with an overall increase in PAX7-expressing MuSCs that retain regenerative capacity after 4 days in culture [10]. Similarly, 10 µM C10 results in poor expansion of MuSCs during ex vivo culture (Figure 2E), likely because P-eIF2α levels are too high (Figure 2C,D). We show that reducing C10 to 5 µM is required for efficient MuSC expansion ex vivo (Figure 2F). MuSCs cultured in the presence of 5 µM C10 retain regenerative capacity as demonstrated by their contribution to dystrophin positive myofibres and the MuSC pool after engraftment into the Dmdmdx mouse model of Duchenne muscular dystrophy (Figure 4). These studies demonstrate that sal003 can be chemically modified to improve and refine MuSC culture conditions for the purpose of cell based therapies for muscle disease. We also show that 5 µM C10 is more efficient than sal003 to expand and serially passage MuSCs isolated from both wild-type and Dmdmdx mice. Starting with an initial population of 7500 fresh isolated MuSCs, we generate millions of wild-type and Dmdmdx myoblasts after 4 passages, greater than fifty percent of which retain PAX7 expression after two passages. Our future studies will ask whether our refined C10 culture conditions can enable genetic correction of the Dmd mutation, and whether corrected MuSCs retain regenerative capacity after engraftment into Dmdmdx mice.
We also demonstrate that MuSCs cultured in the presence of C10 eventually differentiate and generate large myotubes with a greater fusion index. However these large myotubes are continually associated with an increased number of PAX7(+) nuclei, suggesting the effect of C10 on self-renewal of MuSCs during extended culture is maintained. These studies have potential implications for maintaining a reliable source of muscle progenitors for investigation, drug screening, and bioengineering skeletal muscle to model muscle disease.
Here we have reported a novel compound that expands wild-type and Dmdmdx MuSCs ex vivo, enabling their engraftment in vivo and passaging. Further optimization of sal003/C10 analogs may facilitate the development of cell-based therapies for muscle disease, or potentially overcome defects in endogenous MuSC expansion in vivo.
Supplementary File 1: Supplementary Figure S1 (PDF, 2.34 MB)
Supplementary File 2: Supplementary Information (PDF, 5.53 MB)
GL, CC and JL designed the study. GL, SJ and CC performed experiments. MH and OM synthesized novel chemical analogs of sal003. GL and CC analyzed the data. GL and CC wrote the paper with input from all authors.
The authors declare that they have no conflicts of interest.
This research was funded by the Muscular Dystrophy Association (MDA 35129), Canadian Institutes of Health Research (CIHR 399258) and the Stem Cell Network.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
Lean G, Halloran M, Mariscal O, Jamet S, Lumb JP, Crist C. Ex vivo expansion of skeletal muscle stem cells with a novel small compound inhibitor of eIF2α dephosphorylation. Regen Med Front. 2019;1:e190003. https://doi.org/10.20900/rmf20190003

Copyright © 2020 Hapres Co., Ltd. Privacy Policy | Terms and Conditions